
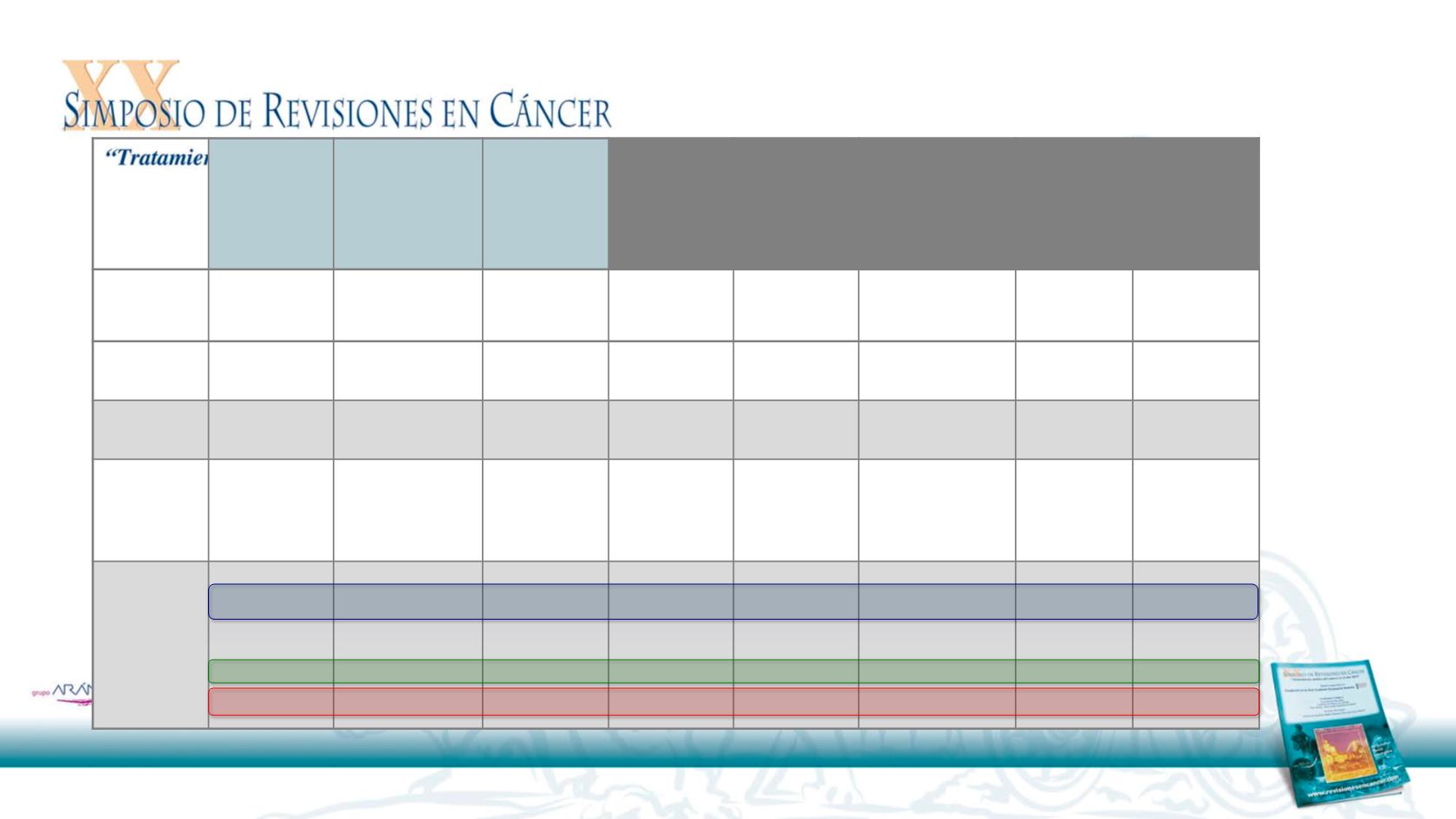
Randomized trials In NSCLC
Main activity data in 2nd line setting
Garon GB et al.
Lancet.
2014. Reck et al. Lancet Oncol 2014. Soria JC et al. Lancet Oncol 2015. Bramer J et al.
NEJM.
2015. Borgahei et al .
Herbst R. Lancet 2015, Fehrenbacher L et al. Lancet 2016. Barlesi F et al. ESMO 2016
Docetaxel+R
amucirumab
vs Docetaxel
Revel Phase
III
Docetaxel+Ninte
danib vs
Docetaxel
LumeLung 1
Phase III
Afatinib vs
Erlotinib
Lux-LUNG 8
Phase III
Nivolumab
vs Docetaxel
CheckMate
017 Phase III
Nivolumab
vs Docetaxel
CheckMate
057 Phase III
Pembrolizumab
vs Docetaxel
KeyNote 010
Phase III
Atezolizuma
b vs
Docetaxel
Poplar
Phase II
Atezoliuzmab
vs Docetaxel
OAK Phase III
Line of T
2nd 100%
2nd 100%
2nd 100%
2nd 100%
2nd line 100% 2nd line 65%, 3rd
line 35%
2nd 70% 3rd
20%m > 3
10%
2nd 75% 3rd
25%
ORR
23 vs 14%
4.7% vs 3.6%
5.5% vs 2.8%
20% vs 9%
19% vs 12%
18% vs 18%
15% vs 15%
14 vs 13%
PFS m
4.5 vs 3
4 vs 2.8
2.6 vs 1.9
3.5 vs 2.8
2.3 vs 4.2
3.9 vs 4 vs 4
2.7 vs 3
2.8 vs 4
OS m
10.4 vs 9.1
9.5 vs 8.2
(SCC)
12.6 vs 10.3
(Adenocarcinoma)
7.9 vs 6.8
9.2 vs 6
12.2 vs 9.4m
10.4-12.7 vs 8.5
12.7 vs 9.7
10.1 vs 8.6
(SCC)
13.8 vs 9.6
HR OS
HR PDL1-
HR PDL1+
0-86
0.883 (SCC)
NA
NA
0.83
NA
NA
0.81
NA
NA
0.62
0.70
0.50
0.73
0.9
0.40
0.71
NA
0.54
0.73
1.09
0.49
0.73
0.75
0.41









