
13
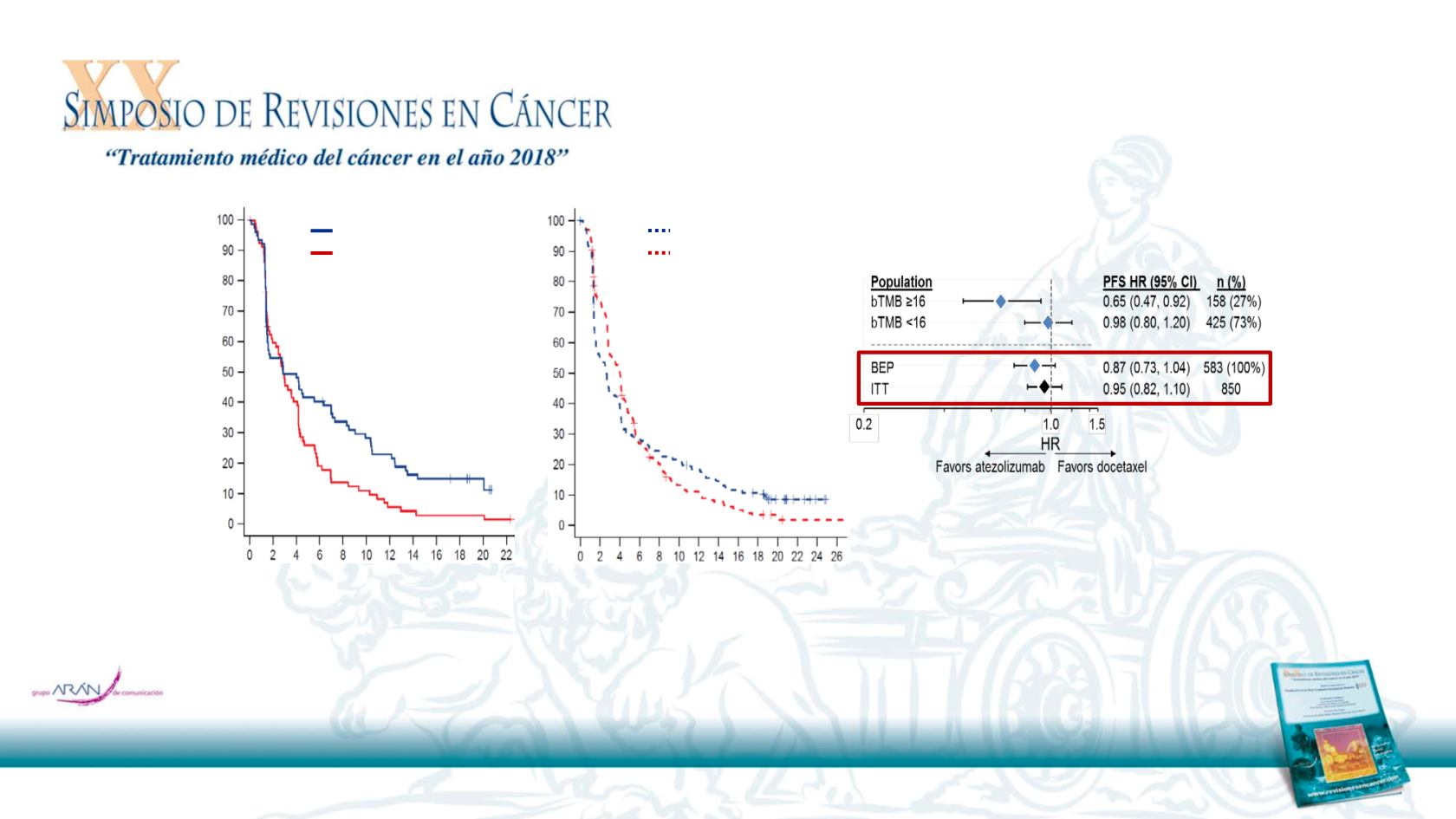
Atezolizumab PFS benefit in bTMB
subgroups validated in the OAK study
BEP, biomarker-evaluable population; HR, hazard ratio; ITT, intention-to-treat.
Progression-Free
Survival (%)
•
The bTMB ≥16 population accounted for 27% of the BEP (N = 158)
•
PFS benefit with atezolizumab versus docetaxel was observed in the bTMB ≥16 subgroup
•
No prognostic effect was observed: patients with bTMB ≥16 did not have improved
PFS compared with patients with bTMB <16 in the docetaxel arm
Interaction
P
= 0.036
Progression-Free
Survival (%)
bTMB ≥16
bTMB <16
Atezolizumab (N = 216)
Docetaxel (N = 209)
+ Censored
Atezolizumab (N = 77)
Docetaxel (N = 81)
+ Censored
Months
Months
Gandara et al. ESMO 2017.









