
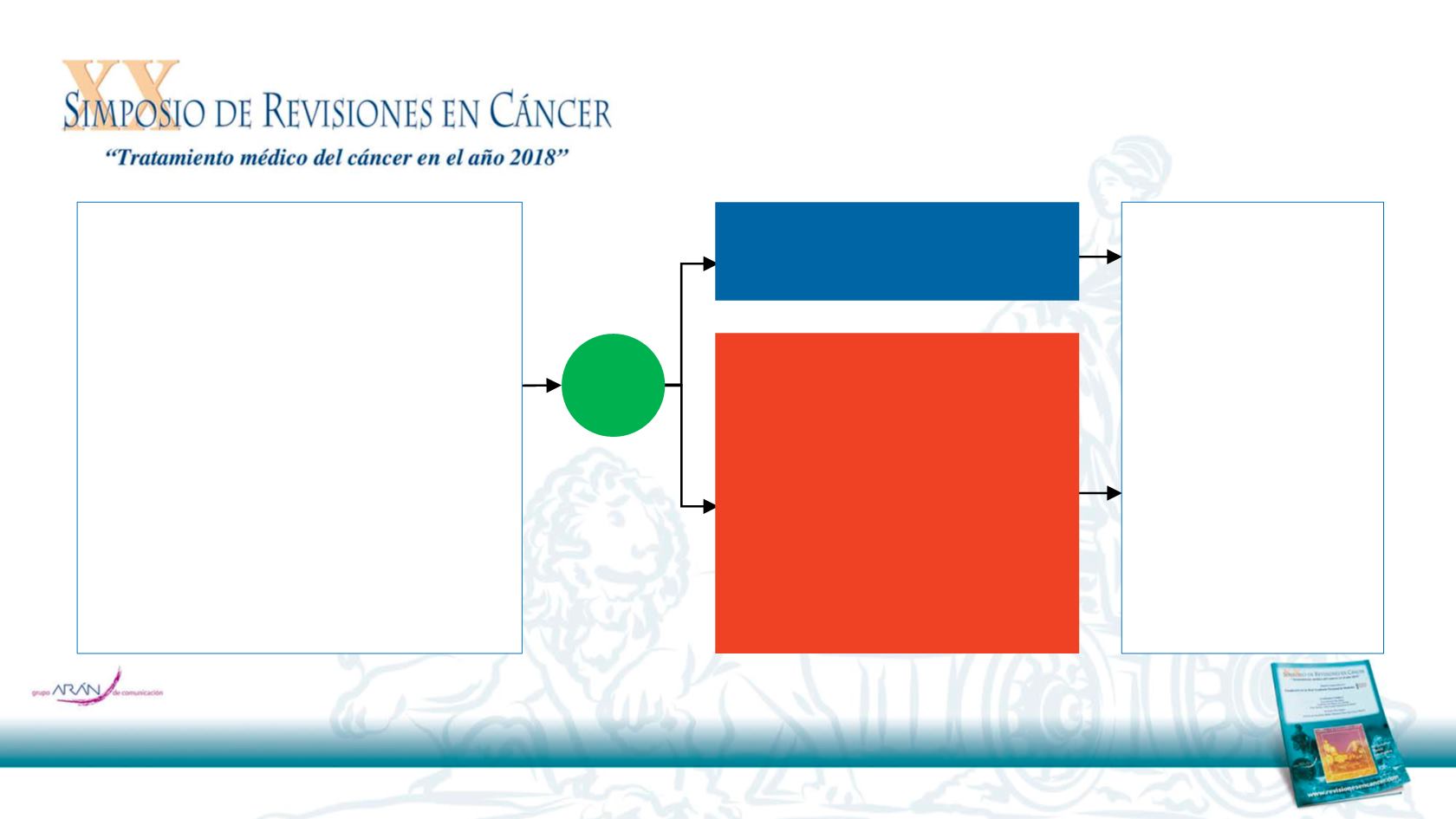
CheckMate 141 Study Design
7
R
2:1
Nivolumab
3 mg/kg IV Q2W
Investigator’s Choice
•
Methotrexate 40 mg/m²
IV weekly
•
Docetaxel 30 mg/m² IV
weekly
•
Cetuximab 400 mg/m² IV
once, then 250 mg/m²
weekly
Key Eligibility Criteria
•
R/M SCCHN of the oral cavity, pharynx,
or larynx
•
Progression on or within 6 months of
last dose of platinum-based therapy
•
Irrespective of no. of prior lines of
therapy
•
Documentation of p16 to determine
HPV status (oropharyngeal)
•
Regardless of PD-L1 status
a
Stratification factor
•
Prior cetuximab treatment
Primary endpoint
•
OS
Other endpoints
•
PFS
•
ORR
•
Safety
•
DOR
•
Biomarkers
•
Quality of life
a
Tissue required for testing.
DOR = duration of response; IV = intravenous; ORR = objective response rate; PFS = progression-free survival; Q2W = once every 2 weeks; R = randomized. Clinicaltrials.gov
NCT02105636.









