
• Atezolizumab (anti–PD-L1) has demonstrated OS benefit over docetaxel (HR, 0.73 [95% CI:
0.53, 0.99]) in a randomized Phase II study, POPLAR, in patients with advanced NSCLC
1
− This benefit has been confirmed in the randomized Phase III study OAK
2
• This presentation describes survival results from POPLAR after a minimum of 3 years follow-up
−
Data cutoff:
7 April 2017;
Median follow-up:
38 months
POPLAR: a randomized Phase II study of atezolizumab
vs docetaxel in NSCLC
IC, tumor-infiltrating immune cells.
a
Tumors were prospectively evaluated for PD-L1 expression using the VENTANA SP142 IHC assay.
References: 1.
Fehrenbacher L, et al
. Lancet
, 2016.
2.
Rittmeyer A, et al.
Lancet
, 2017.
Park, et al. WCLC 2017
13
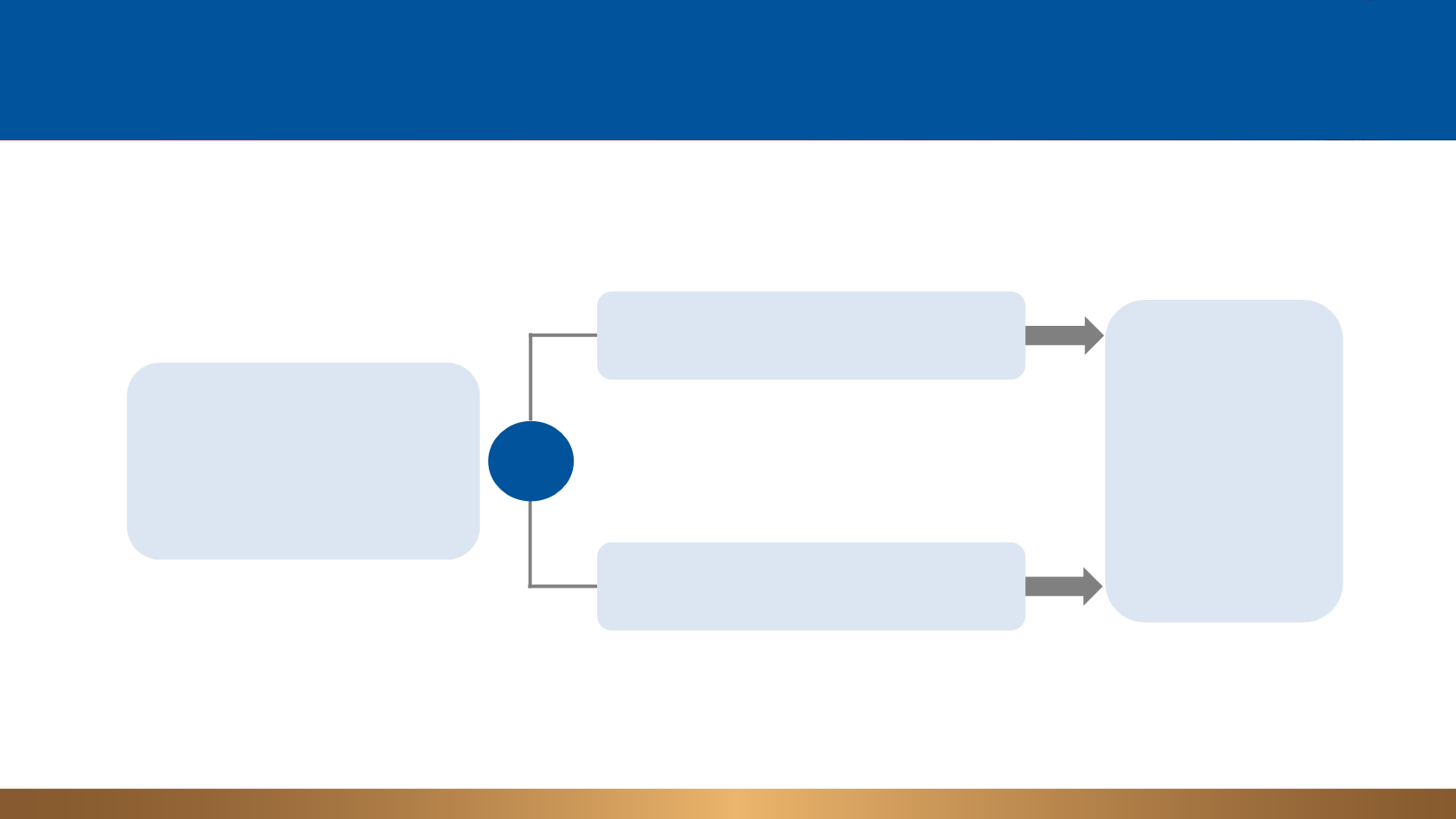
Metastatic or locally
advanced NSCLC (2L/3L)
Disease progression on a prior
platinum therapy
N = 287
Atezolizumab
1200 mg IV q3w
until loss of clinical benefit
Docetaxel
75 mg/m
2
IV q3w
until disease progression
POPLAR (NCT01903993)
Primary Endpoint
OS in ITT and
PD-L1 subgroups
Stratification Factors
• PD-L1 IC expression (0 vs 1 vs 2 vs 3)
a
• Histology (squamous vs non-squamous)
• Prior chemotherapy regimens (1 vs 2)
R
1:1









