
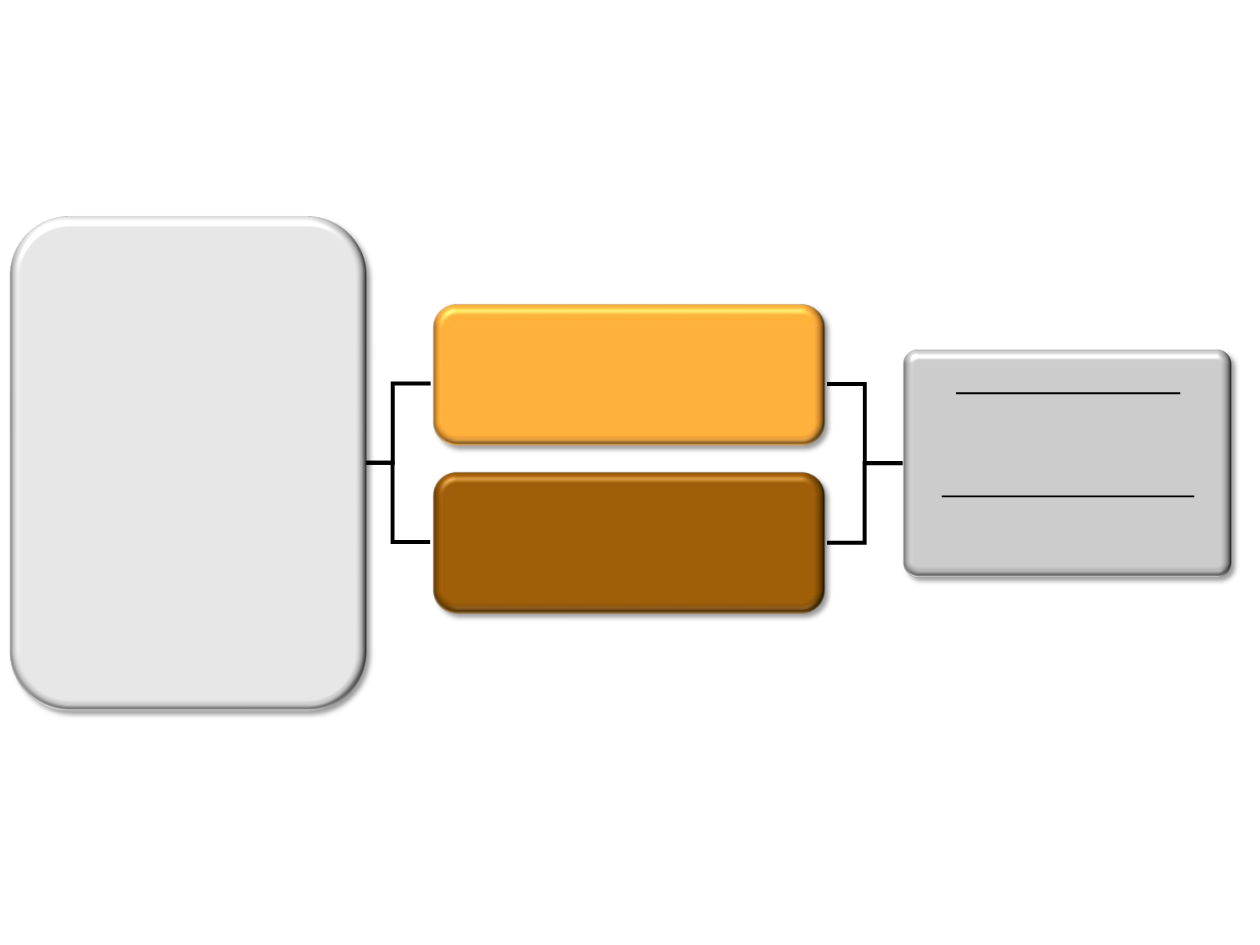
Pivotal BOLERO-2 study: exemestane
±
everolimus
in ABC progressing after NSAIs
•
Stratification
1. Sensitivity to prior hormonal therapy
2. Presence of visceral disease
•
No cross-over
Everolimus 10 mg/day +
Exemestane 25 mg/day
(n = 485)
Placebo +
Exemestane 25 mg/day
(n = 239)
Primary endpoint:
PFS
Secondary endpoints:
OS, ORR, CBR, safety,
QOL, bone markers
ANA, anastrozole; CBR, clinical benefit rate; HER2, human epidermal growth factor receptor; HR+, hormone receptor-positive; LET, letrozole;
NSAI, nonsteroidal aromatase inhibitor; ORR; overall response rate; OS, overall survival; PFS, progression-free survival; PMW, postmenopausal women;
QOL, quality of life.
Baselga J, et al.
N Engl J Med
. 2012;366(6):520-529.
N = 724
PMW with HR+ HER2– ABC
refractory to LET or ANA,
defined as
•
Recurrence during or
within 12 months after end
of adjuvant treatment, or
•
Progression during or
within 1 month after end of
treatment for advanced
disease









