
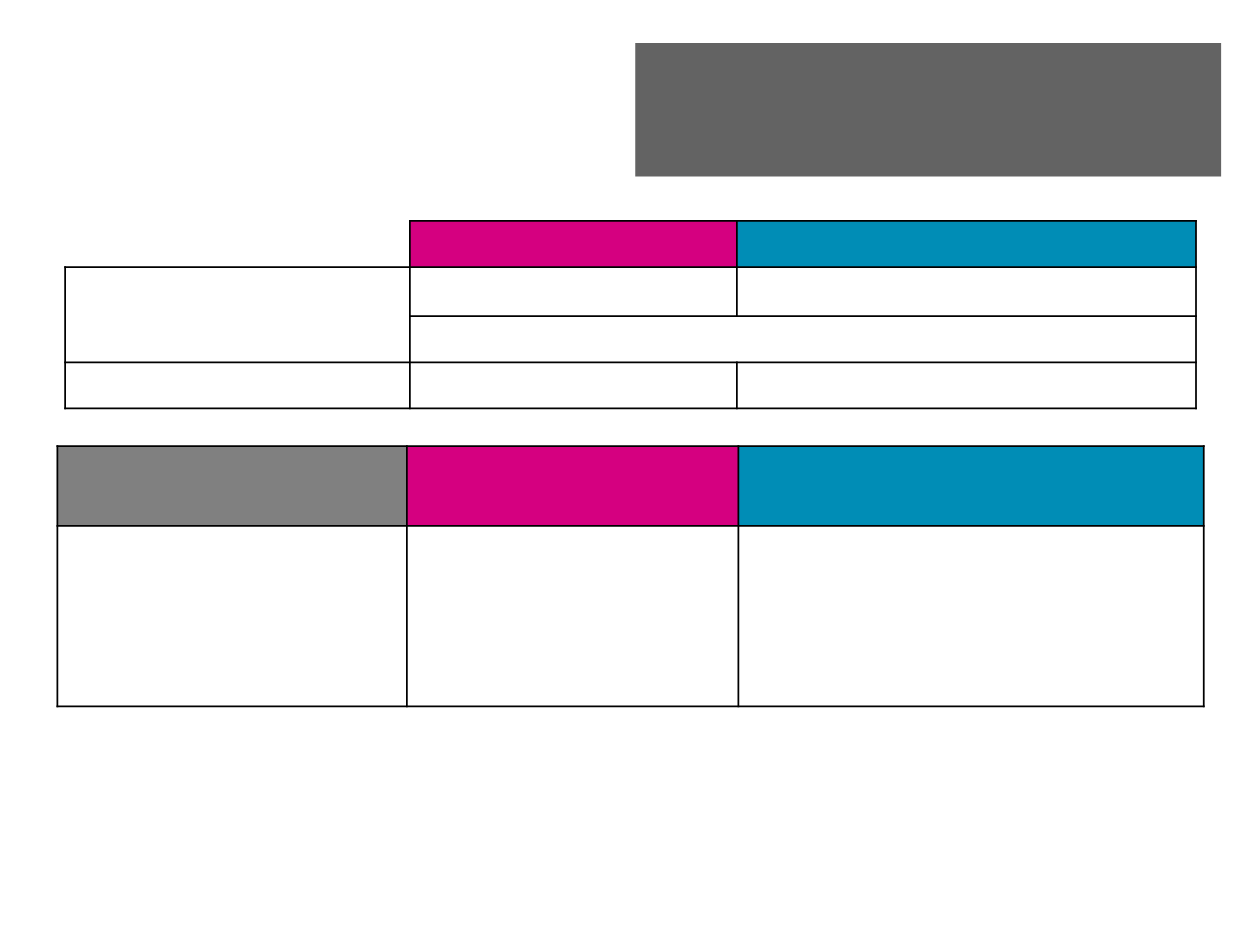
Additional Efficacy Endpoints
Eribulin (n=228)
Dacarbazine (n=224)
PFR
12wks
, % (n) [95% CI*]
OR (95% CI);
P
-value
†
33.3% (76) [27.2-39.9]
28.6% (64) [22.8-35.0]
1.3 (0.8-1.9); 0.253
ORR; % (n)
3.9 (9)
4.9 (11)
Best overall response
Eribulin (n=228)
% (n)
Dacarbazine (n=224)
% (n)
CR
PR
SD
PD
NE/Unknown
0
3.9 (9)
52.2 (119)
39.0 (89)
4.8 (11)
0
4.9 (11)
47.8 (107)
39.3 (88)
8.0 (18)
Tumor assessments based on RECIST v1.1.
1
*95% CI calculated using exact method of binomial distribution.
†
P
-value and odds ratio calculated using the stratified Cochran-Mantel-Haenszel method.
CR, complete response; NE, not evaluable; OR, odds ratio; ORR, objective response rate; PD, progressive disease; PFR
12wks
, progression free rate
at 12 weeks; PR, partial response; SD, stable disease.
1. Eisenhauer et al, Eur J Cancer 2009.
Schöffski P, et al. Presented at ASCO 2015 Annual Meeting, Chicago IL, June 3-7, 2015:abstr LBA10502









