
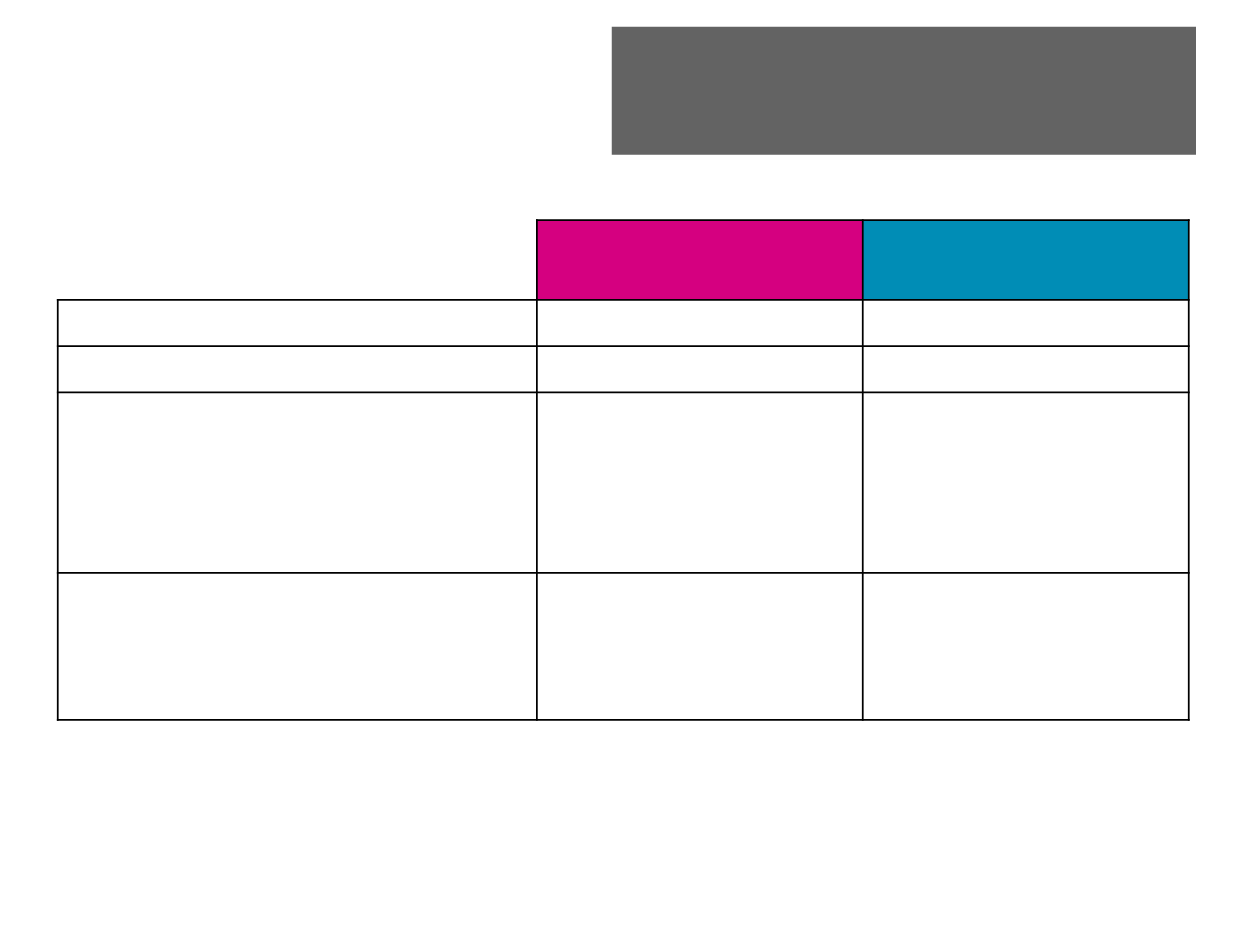
Adverse Events
Eribulin (n=226)
% (n)
Dacarbazine (n=224)
% (n)
Patients with any AE
99.1 (224)
97.3 (218)
Treatment-related AEs*
92.9 (210)
90.6 (203)
AEs with maximum CTCAE grade
≥3
3
4
5
67.3 (152)
38.9 (88)
23.9 (54)
4.4 (10)
56.3 (126)
35.7 (80)
19.2 (43)
1.3 (3)
AEs leading to study drug
Withdrawal
Dose reduction
Dose interruption
7.5 (17)
25.7 (58)
32.7 (74)
4.9 (11)
14.3 (32)
32.1 (72)
*Per investigator assessment.
1. CTCAE v4.02 available at
http://www.acrin.org/Portals/0/Administration/Regulatory/CTCAE_4.02_2009-09-15_QuickReference_5x7.pdf;
accessed May 6, 2015
.
Schöffski P, et al. Presented at ASCO 2015 Annual Meeting, Chicago IL, June 3-7, 2015:abstr LBA10502









