
*Dacarbazine starting dose selected by the local investigator at study initiation; †PFR12wks, proportion of patients who were still alive without disease progression at 12
weeks from randomization.
CR, complete response; CTCAE, Common Terminology Criteria for Adverse Events; IV, intravenous; OS, overall survival; PR, partial response; RECIST, Response Evaluation
Criteria in Solid Tumors.
1. Schöffski P, et al. Presented at ASCO 2015 Annual Meeting, Chicago IL, June 3-7, 2015:abstr LBA10502
2. Schöffski P, et al. Lancet. 2016 Apr 16;387(10028):1629-37
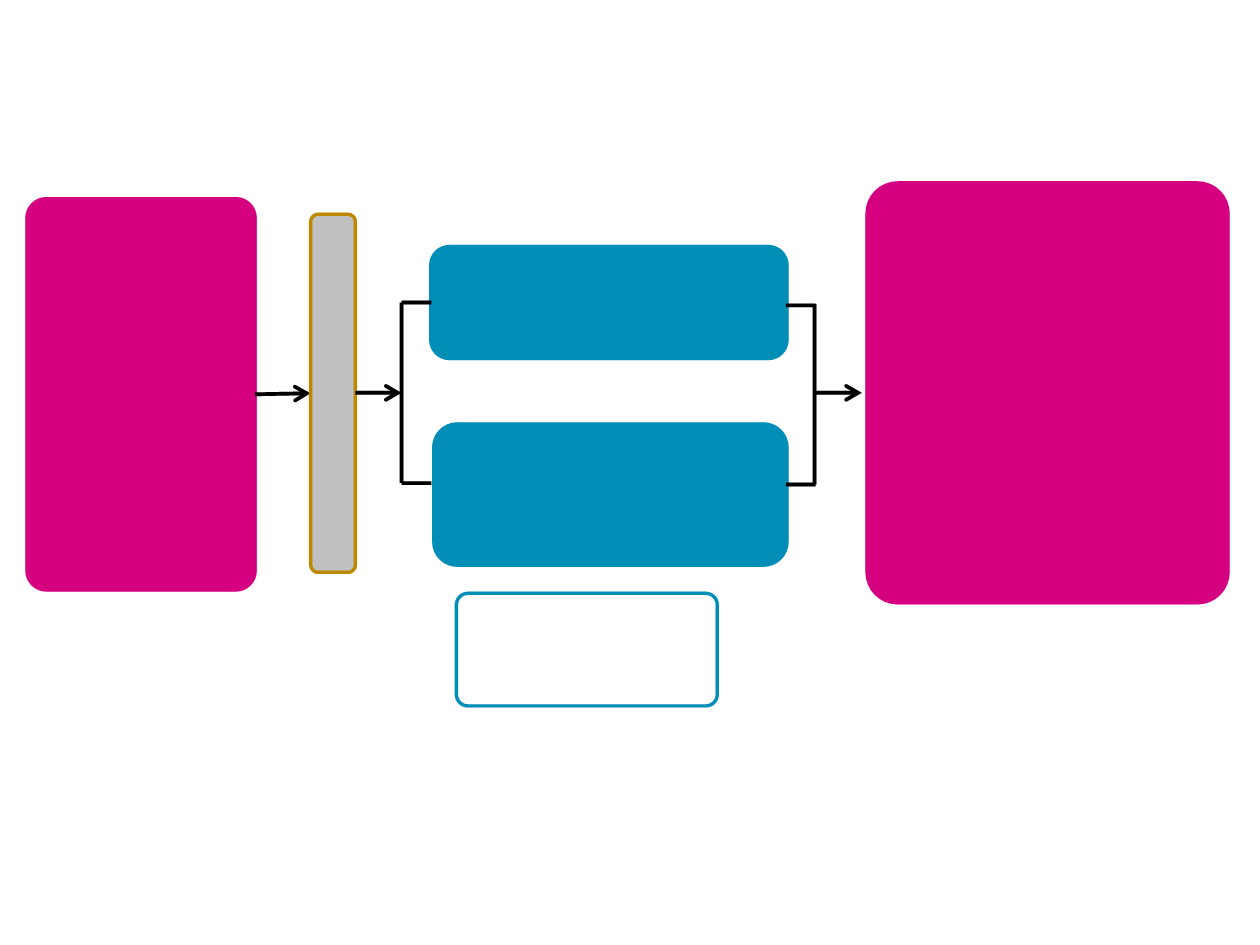
Select eligibility
criteria
•LMS or ADI of high
or intermediate grade
•≥2 prior regimens for
advanced disease
•Measurable disease
(RECIST v1.1)
1
Eribulin
1.23 mg/m
2
IV
Days 1 and 8 every 21 days
R
A
N
D
O
M
I
Z
E
Dacarbazine*
850, 1000, or 1200 mg/m2 IV Day 1
every 21 days
n=224
Primary endpoint
•Overall survival (OS)
Selected secondary
endpoints
•Progression-free survival (PFS)
•Progression-free rate at 12 weeks
(PFR
12wks
)
†
•Safety & tolerability (AE
assessments based on CTCAE
4.02
2
)
•Selected exploratory endpoints
•Objective response rate (ORR;
CR or PR)
•Health-related quality of life
N=452
1:1
Stratification
•
Geographical Region
•
Histology
•
Prior regimens (2; >2)









