
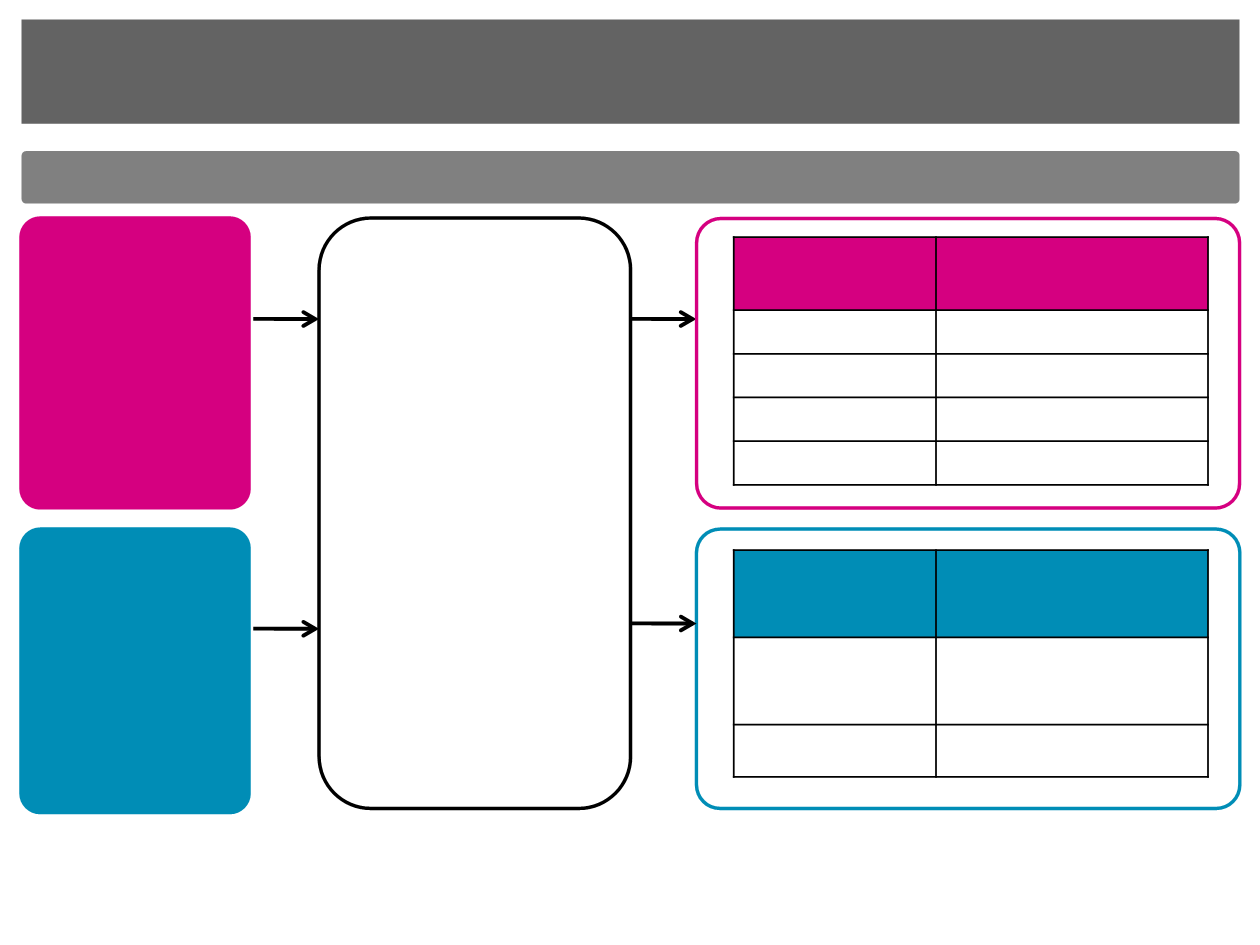
Phase II studies provided the rationale for
Phase III trial in L-type sarcomas
207 Study
1
(N=128)
≤1 previous
combination
chemotherapy or ≤2
single drugs for
advanced disease*
217 Study
2
(N=51)
Japanese patients
with
≥1 standard
chemotherapy
regimen for advanced
disease*
Eribulin 1.23 mg/m
2
Days 1 & 8,
q21 Days
Primary Endpoint:
•
PFR at 12 weeks
Key Secondary
Endpoints:
•
PFS, OS, ORR, AEs
Advanced or metastatic, high or intermediate grade STS*
n=115
Progression-free at 12
weeks, n (%)
Liposarcoma
15/32 (46.9)
Leiomyosarcoma
12/38 (31.6)
Synovial sarcoma
4/19 (21.1)
Other STS
5/26 (19.2)
n=51
Progression-free at 12
weeks, n (%)
Liposarcoma/
Leiomyoscaroma
21/35 (60.0)
Other STS
5/16 (31.3)
AEs = adverse events; ORR = overall response rate; OS = overall survival; PFS = progression-free survival; PFR =
progression-free rate; STS = soft tissue sarcoma. *Key inclusion criteria only shown.
1. Schöffski P, et al. J Lancet Oncol. 2011;12:1045-52;
2. Naito Y, et al. Poster session presented at American Society of Clinical Oncology Annual Meeting; 2014 May 30-June 3; Chicago, IL.









