
Phase I: 1L alectinib + atezolizumab
in
ALK
+ NSCLC
NCT02013219
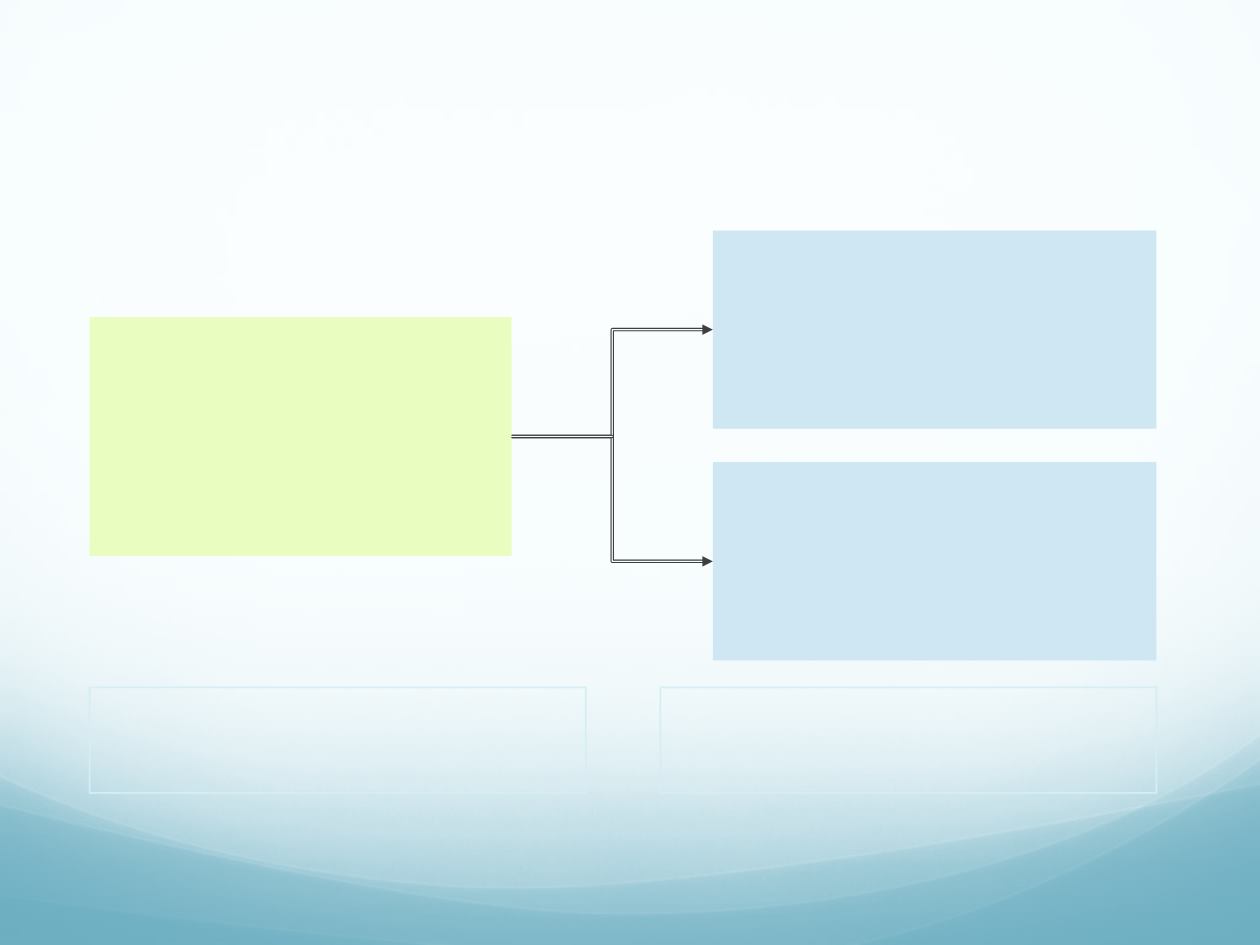
51
•
Locally advanced or metastatic
ALK
+ NSCLC
•
Treatment naïve
•
ECOG PS 0–1
•
Tissue specimen available
(n=26–32)
Dose escalation
Starting doses:
Alectinib 600mg BID
+ atezolizumab (1,200mg IV q3w)
(n=6–12)
Dose expansion
Alectinib 600mg BID
+ atezolizumab (1,200mg IV q3w)
(n=20)
Phase Ib, multicentre, open-label trial
Primary endpoints
•
Safety
•
Recommended phase II dose
Secondary endpoints
•
PK
•
Immunogenic potential of atezolizumab









