
Introduction
1. Johnson TW, et al.
J Med Chem.
2014;57:4720–4744.
2. Zou HY, et al.
Proc Natl Acad Sci U S A.
2015;112:3493–3498.
3. Zou, HY, et al.
Cancer Cell.
2015;28:70–81.
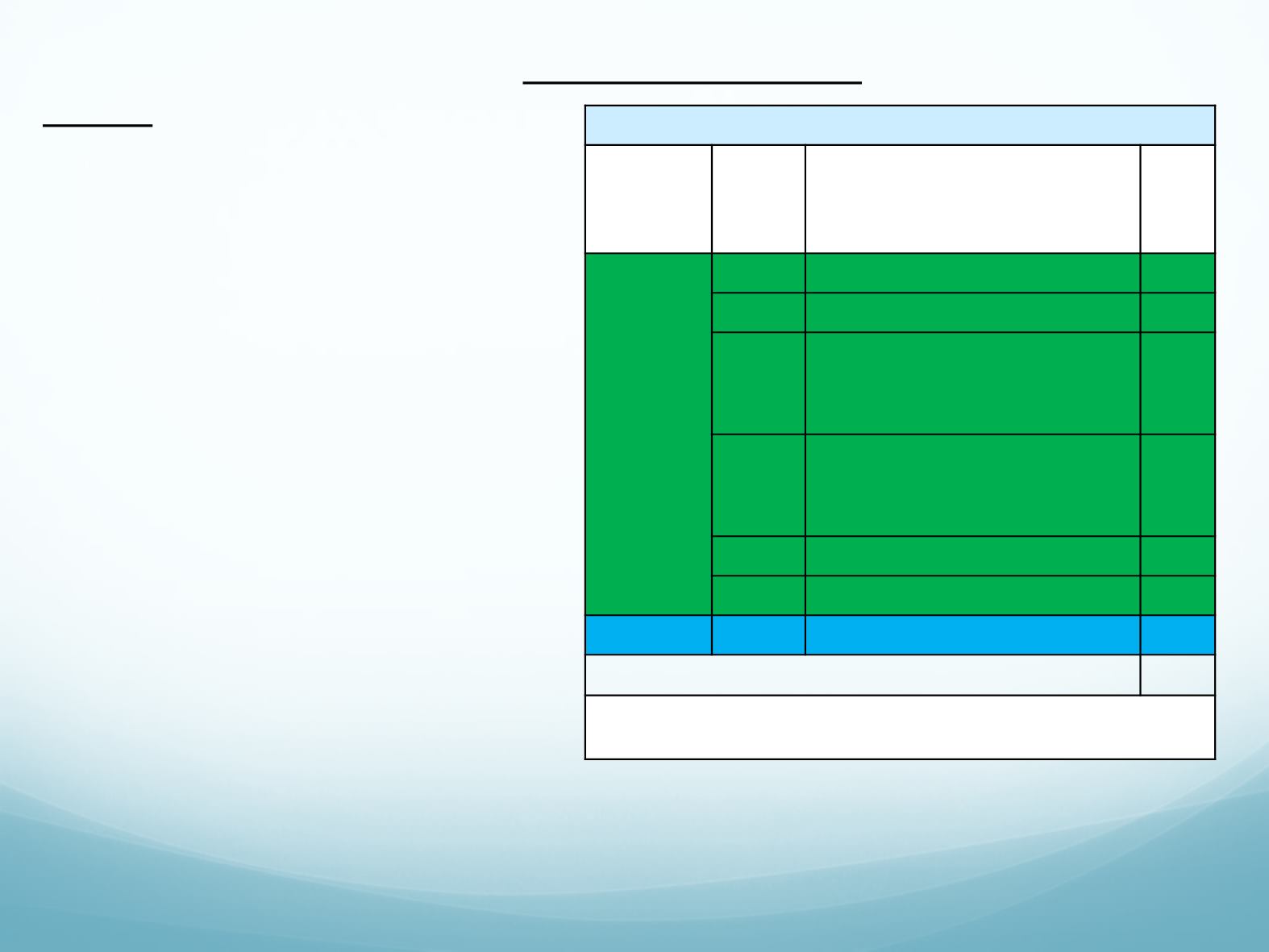
Lorlatinib 100 mg Once Daily (N=275)
a
ALK/RO
S1
Status
Coho
rt
Prior ALK TKI Treatment
Regimen
n
ALK
+
EXP1
Treatment-naïve
30
EXP2
Prior crizotinib only
27
EXP3
A
Prior crizotinib + 1–2 CT
32
EXP3
B
b
Prior non-crizotinib TKI ±
CT
28
EXP4
Two prior TKIs ± CT
65
EXP5
Three prior TKIs ± CT
46
ROS1
+
EXP6
Any
47
Total
275
a
Treatment until PD or unacceptable toxicity; treatment beyond PD allowed if the
patient derived benefit.
b
A patient was excluded from ITT population as ALK+ status was not documented.
Overview
• Lorlatinib is a selective, potent, brain-
penetrant anaplastic lymphoma
kinase (ALK)/c-ros oncogene 1
(ROS1) tyrosine kinase inhibitor (TKI)
active against most known
ALK
kinase domain mutations.
1–3
• Phase 1 of this ongoing study
(NCT01970865) demonstrated that
lorlatinib had robust clinical activity in
patients with ALK
+
/ROS1
+
non-small
cell lung cancer (NSCLC), most of
whom were heavily pretreated and
had central nervous system
metastases.
4
Study Design and Objectives
Primary Objective
• Overall and intra-cranial [IC]
antitumor activity measured as
confirmed overall and IC
response by independent central
review
Select Secondary Objectives
• Safety and tolerability
• Patient-reported outcomes
• Molecular profiling









