
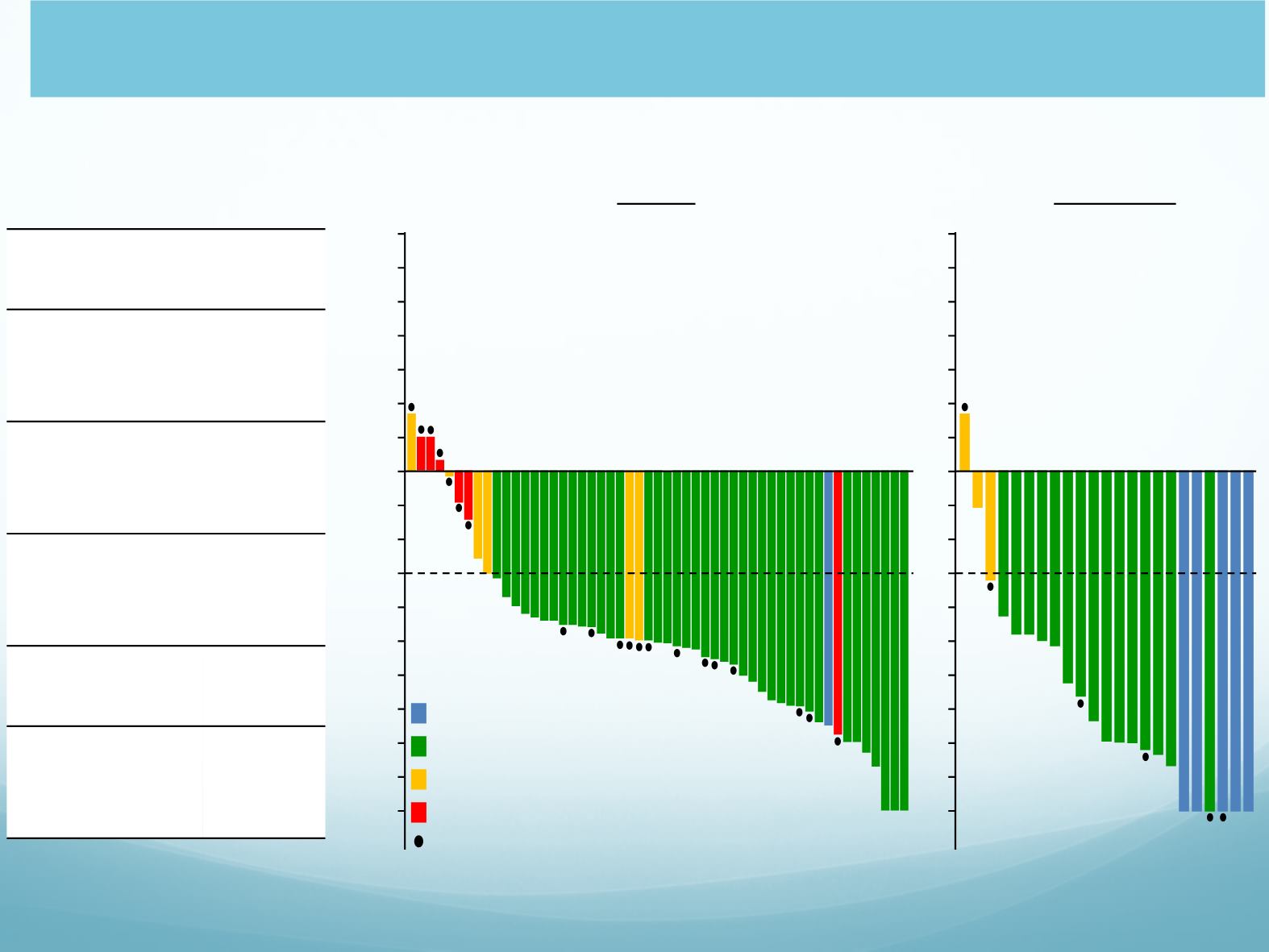
Pooled Efficacy in EXP2 (ALK
+
, Crizotinib Only)
and EXP3A (ALK
+
, Crizotinib + CT) cohorts
70
60
10
0
30
20
50
40
‒10
‒20
‒30
‒40
‒50
‒60
‒70
‒80
‒90
‒100
Best Change From Baseline (%)
EXP2+3
A (n=59)
ORR, n/N (%)
(95% CI)
41/59
(69)
(56, 81)
IC ORR, n/N
(%)
(95% CI)
25/37
(68)
(50, 82)
Median DOR,
mo (95% CI)
NR
(11.1,
NR)
DOR ≥6 mo,
n
⁰
/n (%)
20/41
(49)
Median PFS,
mo
(95% CI)
NR
(12.5,
NR)
• 37 patients (63%) had brain
metastases at baseline.
Off treatment or PD occurred
Complete response
Partial response
Stable disease
Progressive disease (PD)
Overall
a,b
70
60
10
0
30
20
50
40
‒10
‒20
‒30
‒40
‒50
‒60
‒70
‒80
‒90
‒100
Intracranial
a,b
CI, confidence interval; CT, chemotherapy; DOR, duration of response; mo, months; NR, not reached.
a
Patients with at least one on-study target lesion assessment as per independent central review were included. If any procedure was different and not interchangeable from the procedure at screening, the percent change from baseline could not be calculated and is
not displayed.
b
Complete response was defined as the disappearance of all target lesions; when nodal disease was included in target lesions, reversion to normal node size (<10 mm) prevented the percent change from baseline from reaching –100%. Some patients with a total
change from baseline of –100% are shown as partial responses due to the inclusion of non-target lesions in the summary.









