
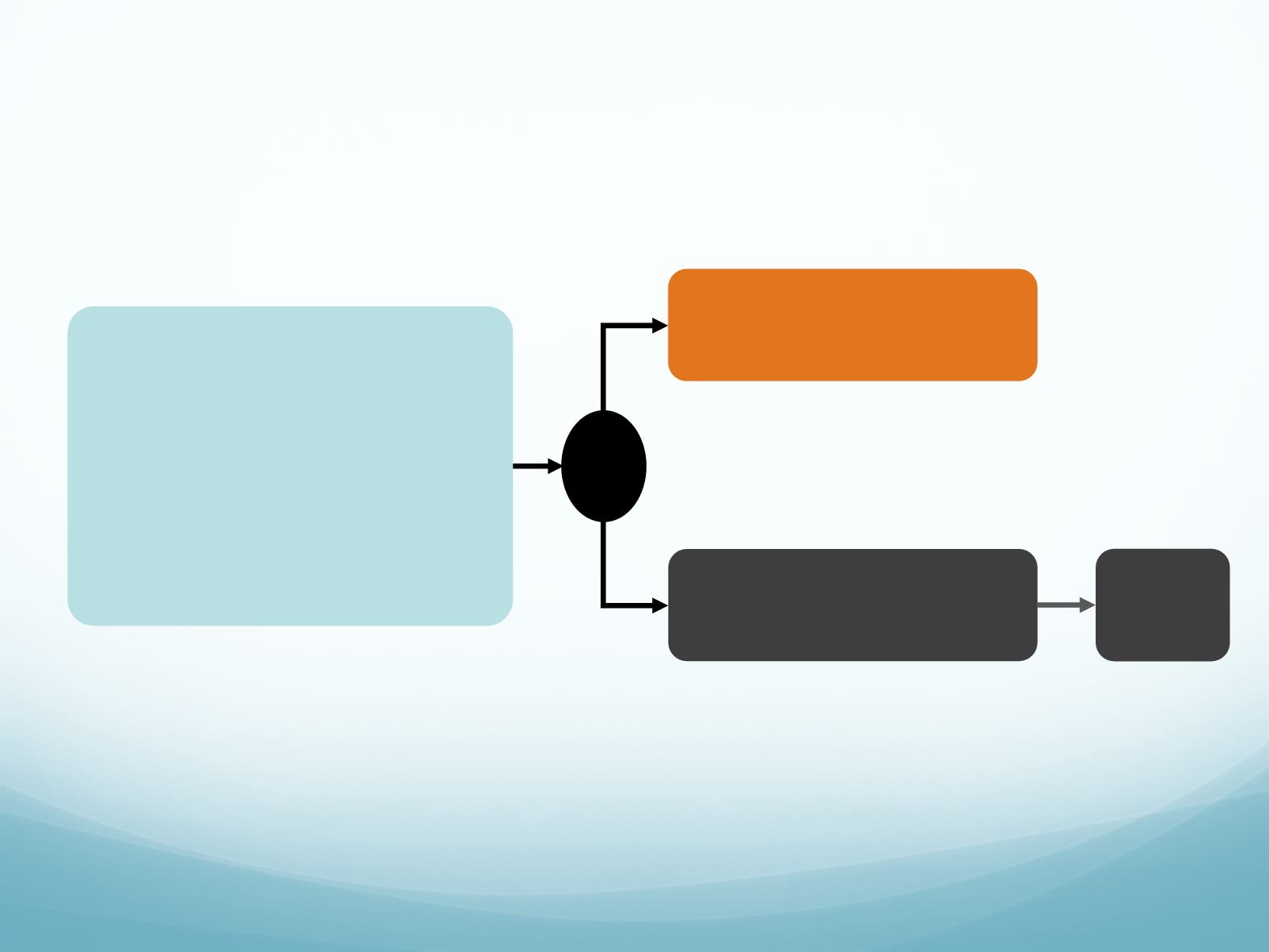
Soria, et al. Lancet 2017
Pemetrexed/cisplatin or
pemetrexed/carboplatin
i.v. q3w
§
R
1:1
Ceritinib 750mg/day
•
Newly diagnosed stage IIIB/IV or
relapsed locally advanced or metastatic
non-squamous NSCLC
•
ALK+
disease according to IHC
•
No previous treatment*
•
≥1 measurable lesion as defined by
RECIST 1.1
(n=376)
Primary endpoint
•
PFS (assessed by BIRC per RECIST 1.1)
Pemetrexed
maintenance
*Exception of neo-adjuvant or adjuvant therapy
§
Cycle = 21 days; Duration = four cycles of treatment (induction) followed by pemetrexed
alone as single agent (maintenance) every 21 days in patients without blinded independent
review committee (BIRC) progressive disease (PD) after induction chemotherapy
Chemotherapy









