
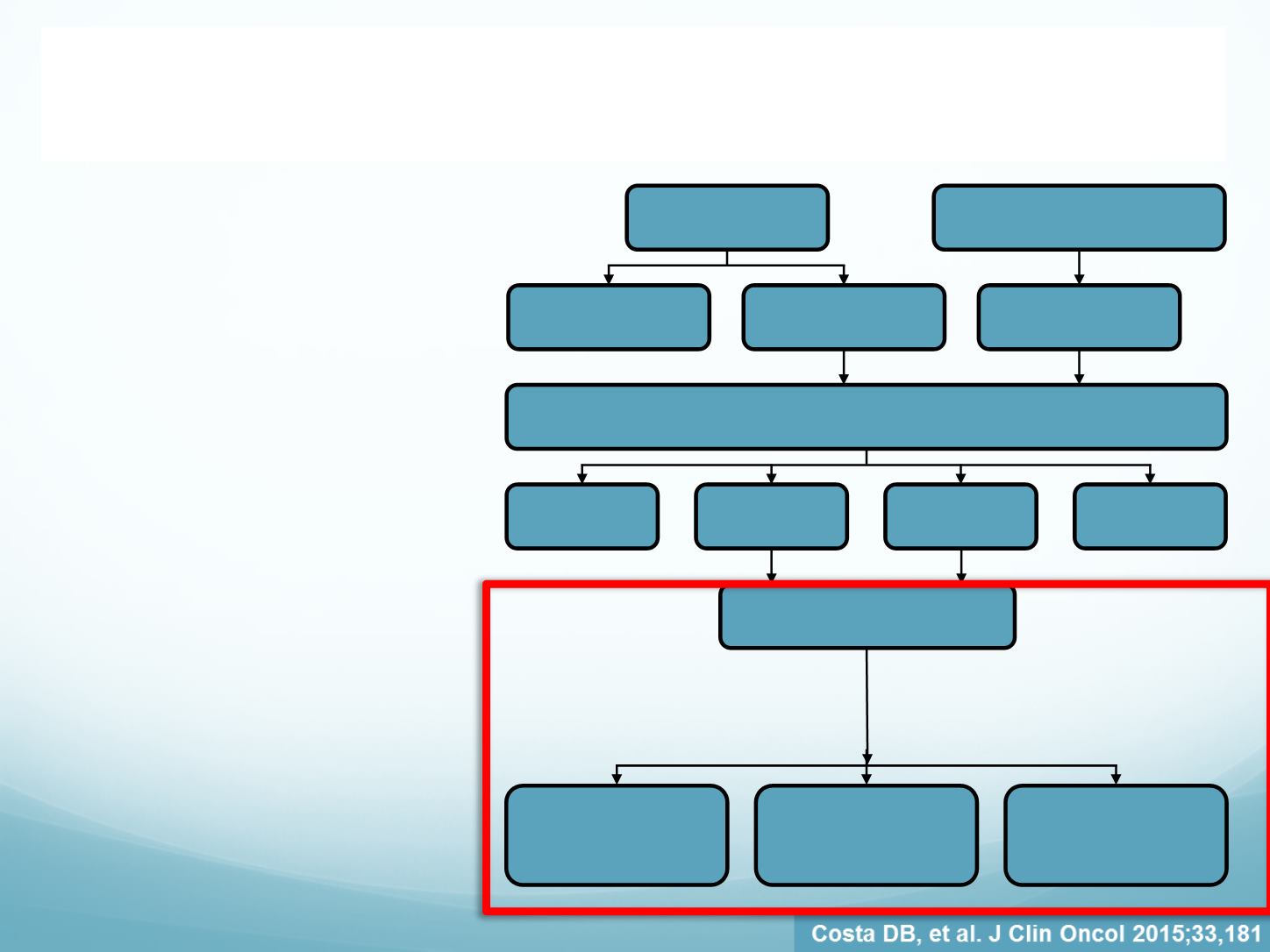
CRIZOTINIB CNS ACTIVITY
888 patients were pooled
from PROFILE 1005 and
1007 as shown
Three patient groups were
defined − those with:
Previously untreated
(no prior radiotherapy)
asymptomatic brain
metastases (12%)
Previously treated (with
intracranial radiotherapy)
asymptomatic brain
metastases (19%)
No detectable brain
metastases at baseline
(69%)
PROFILE 1005
(n=934)
PROFILE 1007 (treated with
crizotinib; n=172)
ALK
-positive by
local tests
(n=127)
ALK
-positive by FDA-
approved FISH test
(n=807)
ALK
-positive by FDA-
approved FISH test
(n=172)
Patients with adequate baseline tumor assessments who:
1. Had ≥2 post-baseline tumor scans (with one ≥6 weeks after treatment start) or
2. Discontinued/progressed/died at anytime after treatment start
Did not meet
criteria
(n=77)
Met criteria
(n=730)
Met criteria
(n=158)
Did not meet
criteria
(n=14)
Pooled PROFILE 1005 and
PROFILE 1007 (n=888)
Previously untreated
asymptomatic brain
metastases
(n=109)
Previously treated
asymptomatic brain
metastases
(n=166)
No detected brain
metastases
(n=613)









