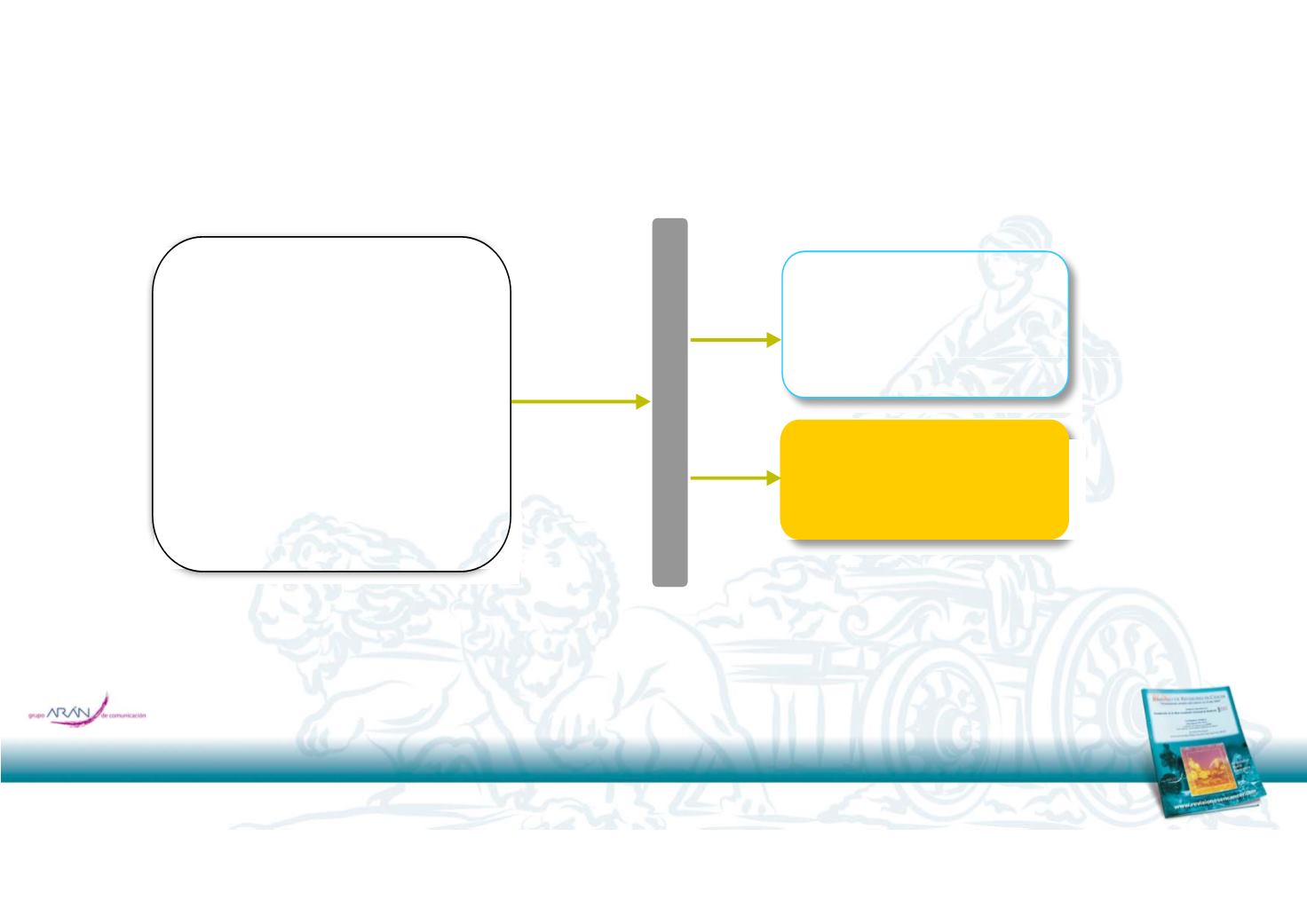
TIVO‐3: Phase 3 trial of Tivozanib vs. Sorafenib as 3
rd
or 4
th
line
Eligibility:
Tivozanib: 1 5 mg
R
A
N
Subjects with
recurrent
metastatic RCC who
have
failed 2
or
3 prior
systemic
regimens,
f
hi h
i l d
VEGFR
.
(3 wk on/
1 wk off)
D
O
M
I
one
o w c nc u es a
TKI
Clear Cell Component
Sorafenib: 400 mg orally
twice daily
Z
A
T
I
N=322
Excluded >3 prior regimens
O
N
Stratification factors:
Primary end point: PFS based on BICR
Secondary end points:
ORR, OS, Safety
•
ECOG PS
(0 vs
1)
•
Prior
therapy with PD‐1/PDL‐1
(Y/N)
EudraCT) Number :2015‐003607‐30; McTigue M, et al. PNAS 2012








