1ª línea: estudios en marcha
Fase Ib: axi/pembro; chemonäive
KEYNOTE 426
PD‐1
+ VEGFR
TK
inhibition
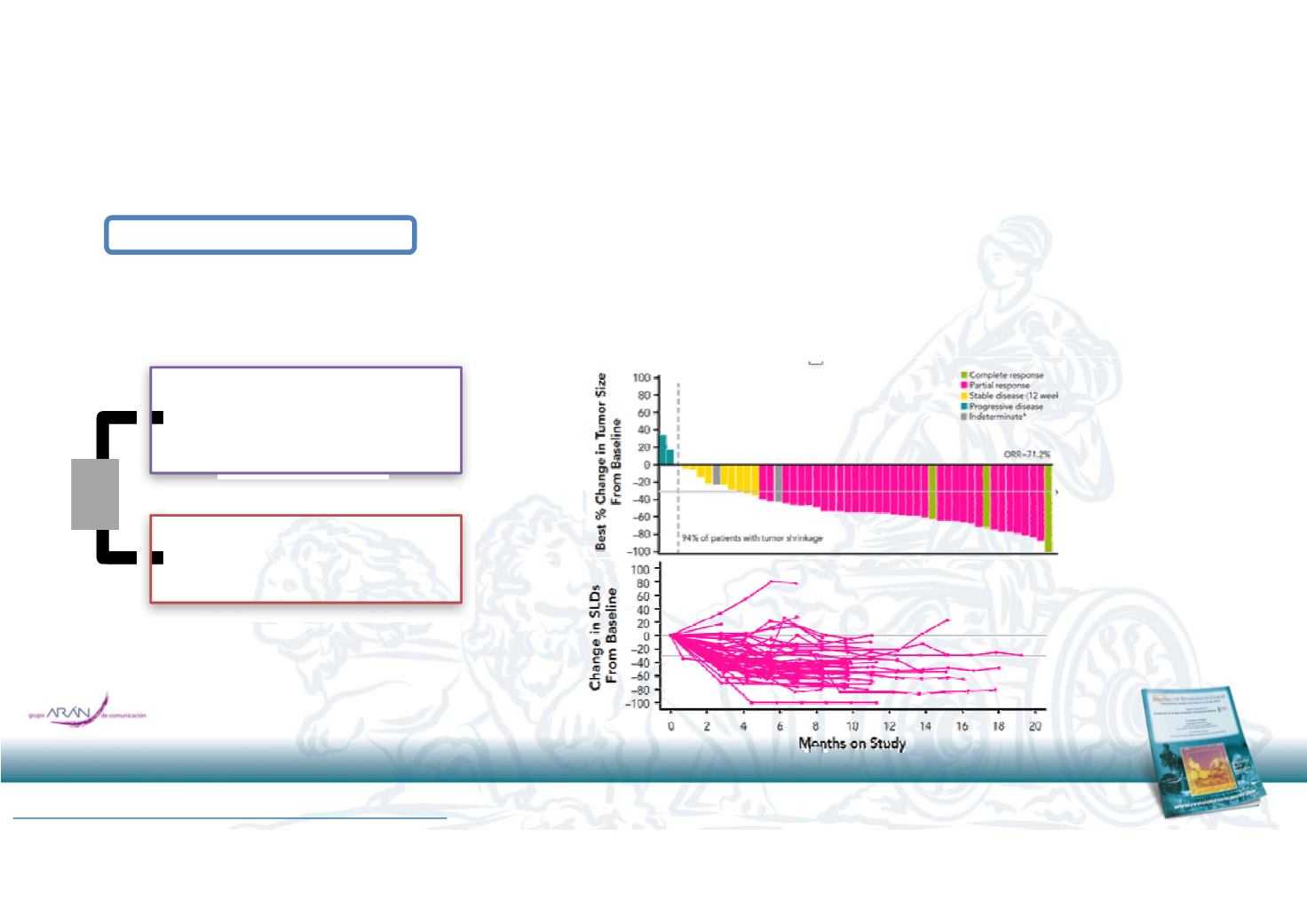
52 pacientes
RR: 73%
PFS 21
Phase III
Sunitinib
meses
50 mg/day
4/2
R
n=840
Axitinib + pembro
5 mg BID + 200 mg
(IV) every 3 weeks
Co-Primary endpoint: PFS, OS
https://clinicaltrials.gov/ct2/show/NCT02853331; Atkins MB, et al. ASCO‐GU 2018 #579








