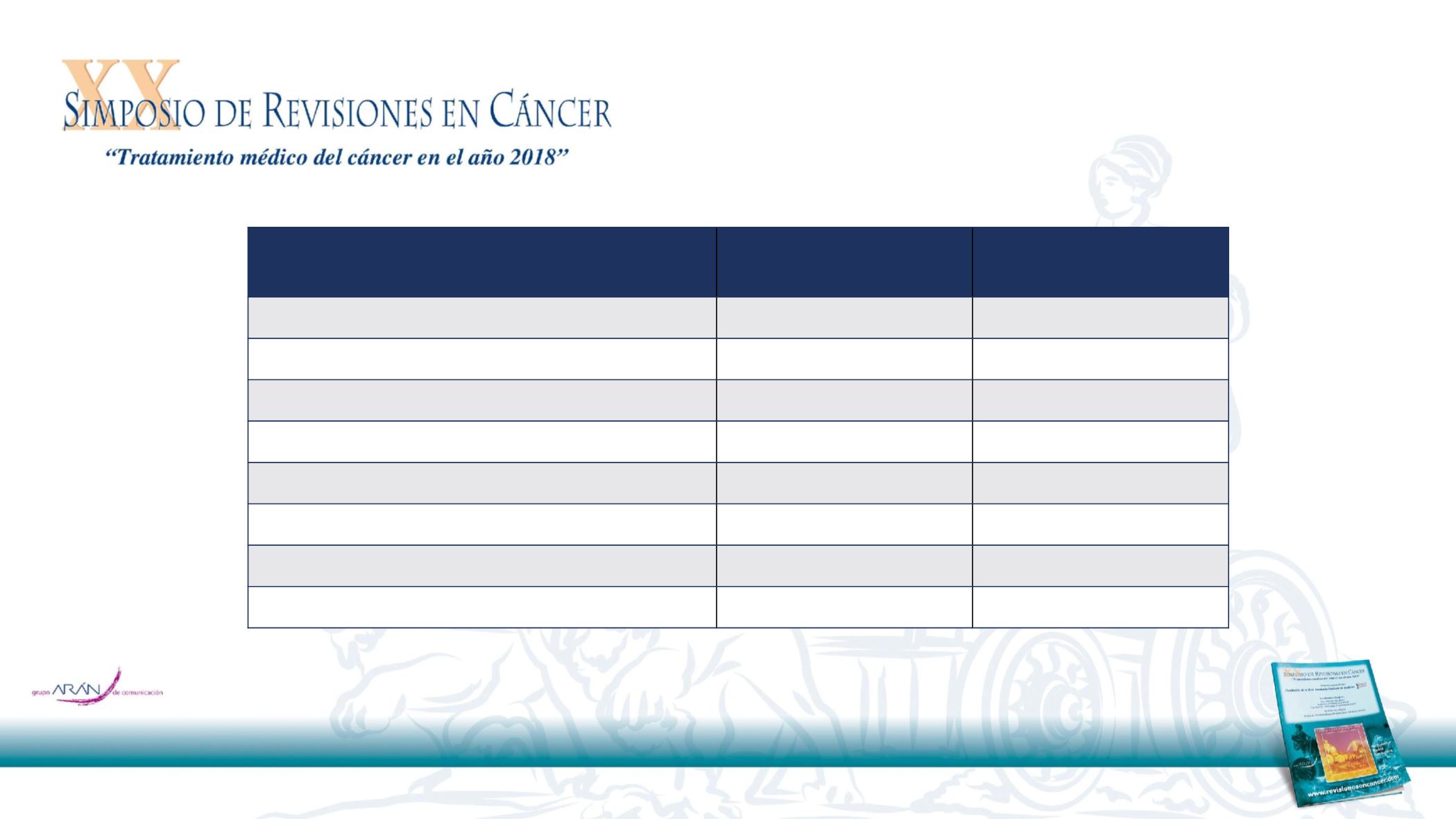
*A patient may have had more than one new lesion site.
†
Includes lesions in: abdominal wall, biliary tract, breast, chest wall, kidney, ovary, pancreas,
pericardium, peritoneal fluid, peritoneum, retroperitoneum, skin, spleen, uterus and other (unspecified). BICR, blinded independent central review; ITT,
intention-to-treat
From Durvalumab after Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer, Antonia SJ, et al, N Engl J Med 2017 Sep 8 [ePub ahead of print] Copyright © (2017) Massachusetts Medical
Society. Reproduced with permission
New lesion site*
Durvalumab
(N=476)
Placebo
(N=237)
Any new lesion, n (%)
97 (20.4)
76 (32.1)
Lymph nodes
27 (5.7)
27 (11.4)
Brain
26 (5.5)
26 (11.0)
Lung
56 (11.8)
41 (17.3)
Liver
9 (1.9)
8 (3.4)
Adrenal
3 (0.6)
5 (2.1)
Bone
8 (1.7)
6 (2.5)
Other
†
9 (1.9)
5 (2.1)








