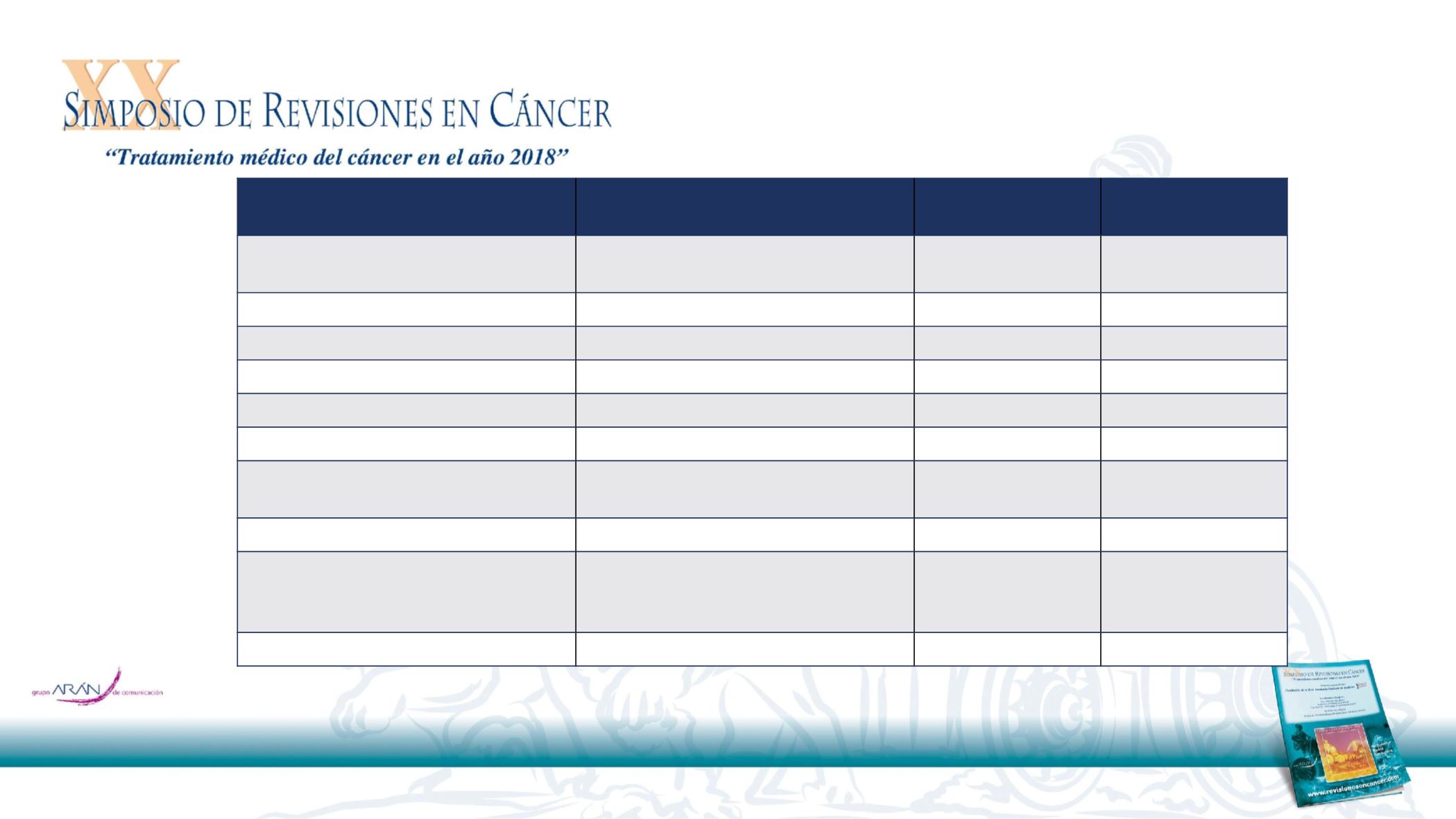
*Not reported or missing (durvalumab, placebo, total): WHO performance status (0.4% each), prior radiotherapy (0.2%, 0.4%, 0.3%).
†
Other: durvalumab, 2.5%; placebo, 2.1%; total, 2.4%.
‡
No sample collected or no valid test result.
¶
Not evaluable/not applicable: durvalumab, 2.3%; placebo,
2.1%; total, 2.2%.
cCRT, concurrent chemoradiation therapy; CR, complete response; ITT, intention-to-treat; PD, progressive disease; PD-L1, programmed cell death ligand-1;
PR, partial response; SD, stable disease; TC, tumor cell; TC ≥25%, ≥25% PD-L1 expression on tumor cells; TC <25%, <25% PD-L1 expression on tumor
cells; WHO, World Health Organization
.
Antonia SJ, et al, N Engl J Med 2017 Sep 8 [ePub ahead of print]
Durvalumab
(N=476)
Placebo
(N=237)
Age
Median (range), years
≥65 years, %
64 (31–84)
45.2
64 (23–90)
45.1
Male, %
70.2
70.0
WHO performance status score, %*
0 / 1
49.2 / 50.4
48.1 / 51.5
Smoking status, %
Current / Former / Never
16.6 / 74.4 / 9.0
16.0 / 75.1 / 8.9
Disease stage, %
†
IIIA / IIIB
52.9 / 44.5
52.7 / 45.1
Histology, %
Squamous / Non-squamous
47.1 / 52.9
43.0 / 57.0
PD-L1 status, %
Known: TC <25% / TC ≥25%
Unknown
‡
39.3 / 24.2
36.6
44.3 / 18.6
37.1
Prior chemotherapy, %
Induction / Definitive cCRT
25.8 / 99.8
28.7 / 99.6
Prior radiotherapy, %*
<54 Gy
54 to ≤66 Gy
>66 to ≤74 Gy
0.6
92.9
6.3
0
91.6
8.0
Best response to prior cCRT, %
¶
CR / PR / SD / PD
1.9 / 48.7 / 46.6 / 0.4
3.0 / 46.8 / 48.1 / 0








