
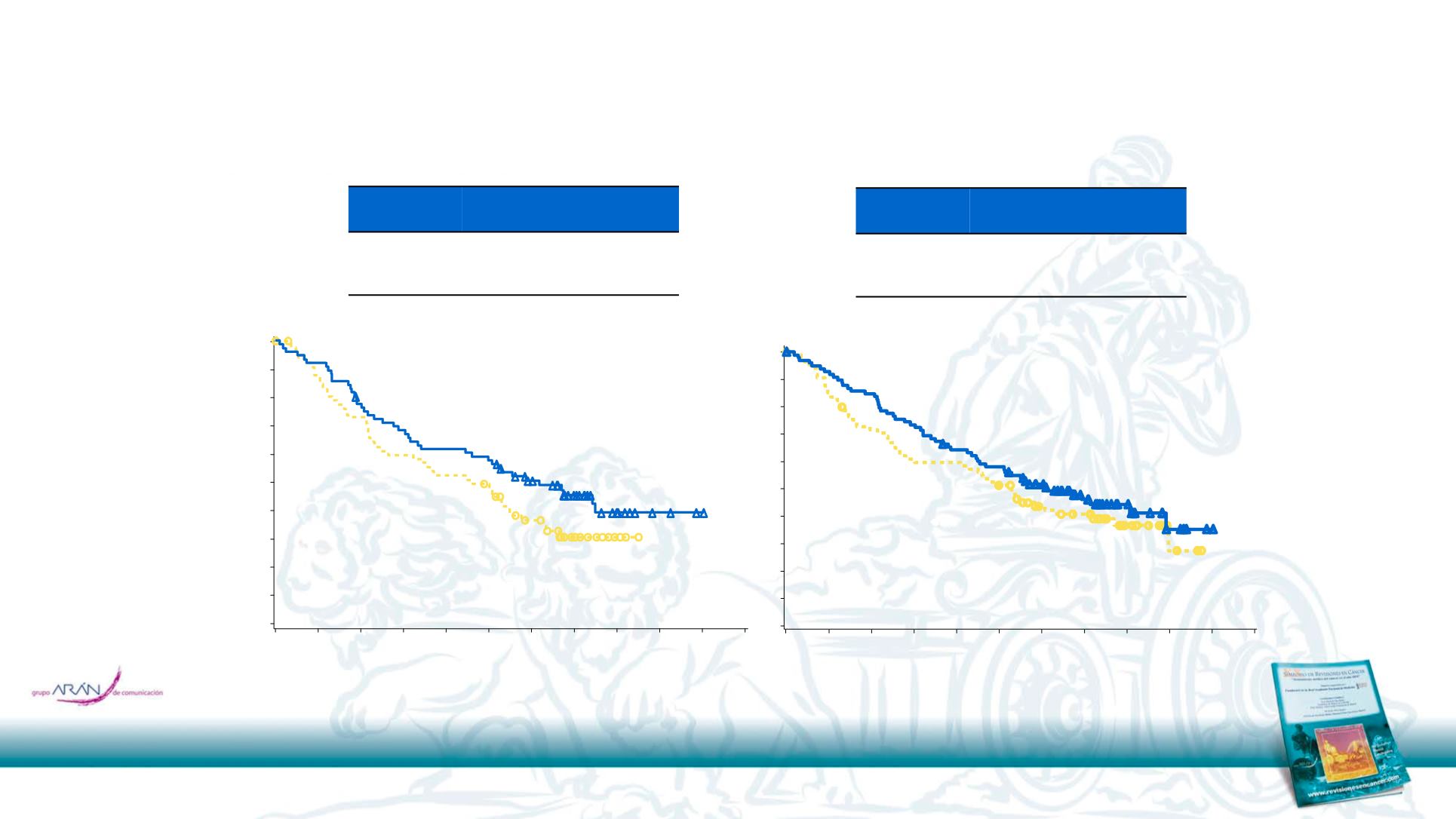
OS by Bone and Liver Metastases in Patients With mRCC
Nivolumab
0.0
0
3
6
12
9
15
Months
18
21
24
27
30
33
0.1
0.8
0.9
100
83
62
43
15
1
87
60
50
30
11
0
Everolimus
0.2
0.3
0.4
0.5
0.6
0.7
1.0
Everolimus
Nivolumab
0
3
6
12
9
15
Months
18
21
24
27
30
33
No. of patients at risk
Nivolumab 76
58
46
32
9
1
Everolimus 70
49
35
20
3
0
Overall Survival (Probability)
0.0
0.1
0.8
0.9
0.2
0.3
0.4
0.5
0.6
0.7
1.0
HR (95% CI
), 0.72 (0.47–1.09)
Median OS, months
(95% CI)
Nivolumab
18.5 (10.2–NE)
Everolimus
13.8 (7.0–16.4)
Bone
HR (95% CI
), 0.81 (0.55–1.18)
Median OS, months
(95% CI)
Nivolumab
18.3 (13.4–26.7)
Everolimus
16.0 (8.4–21.6)
Liver
Based on data cut-off of June 2015.Motzer RJ et al. Oral presentation at ASCO GU 2016.498.









