
Key End Points
Primary: PFS (RECIST v1.1 per blinded, independent central review)
Secondary: OS, ORR, safety
Exploratory: DOR
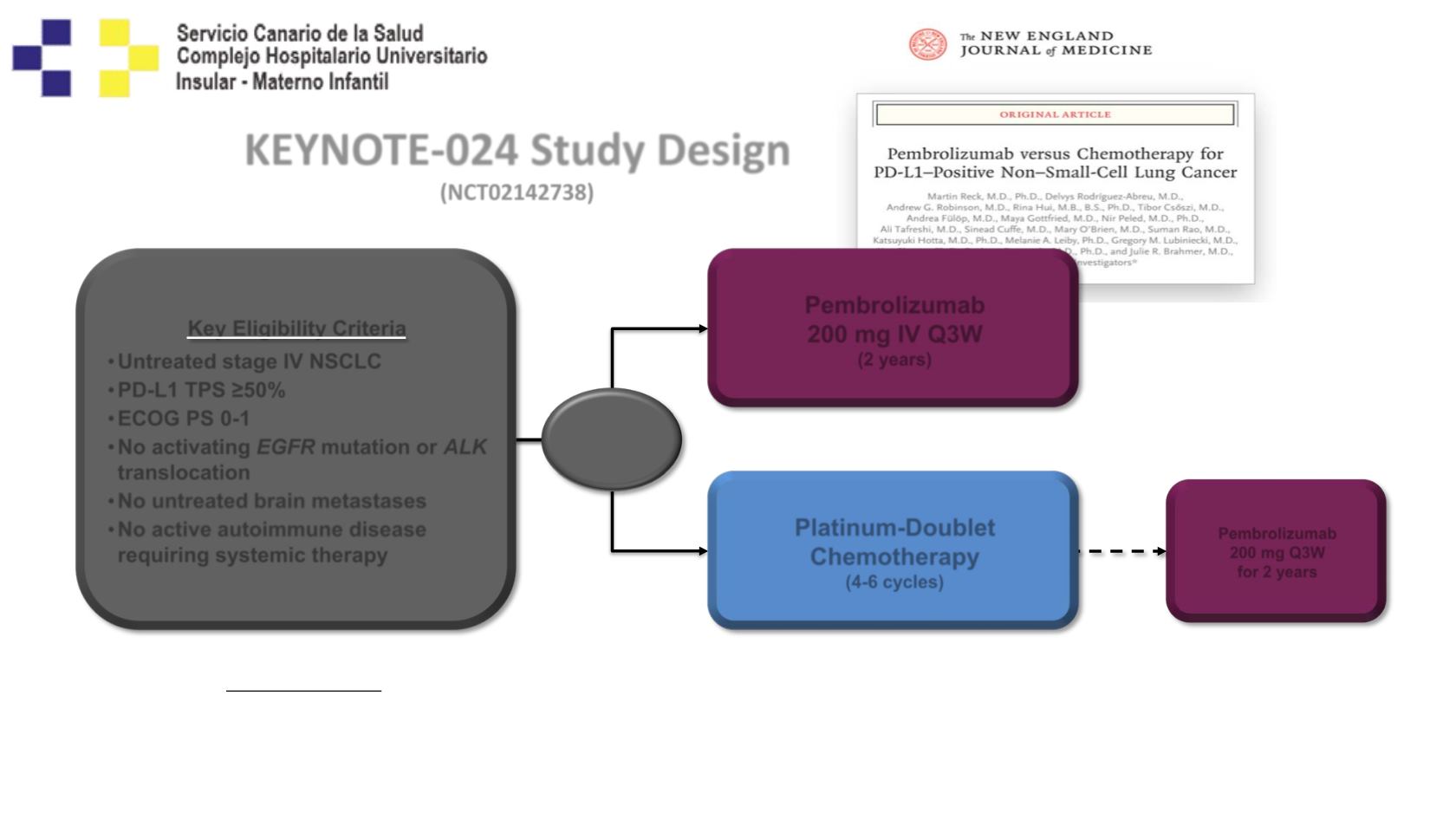
KEYNOTE-024 Study Design
(NCT02142738)
Key Eligibility Criteria
•
Untreated stage IV NSCLC
•
PD-L1 TPS ≥50%
•
ECOG PS 0-1
•
No activating
EGFR
mutation or
ALK
translocation
•
No untreated brain metastases
•
No active autoimmune disease
requiring systemic therapy
Pembrolizumab
200 mg IV Q3W
(2 years)
R (1:1)
N = 305
PD
a
Pembrolizumab
200 mg Q3W
for 2 years
Platinum-Doublet
Chemotherapy
(4-6 cycles)









