
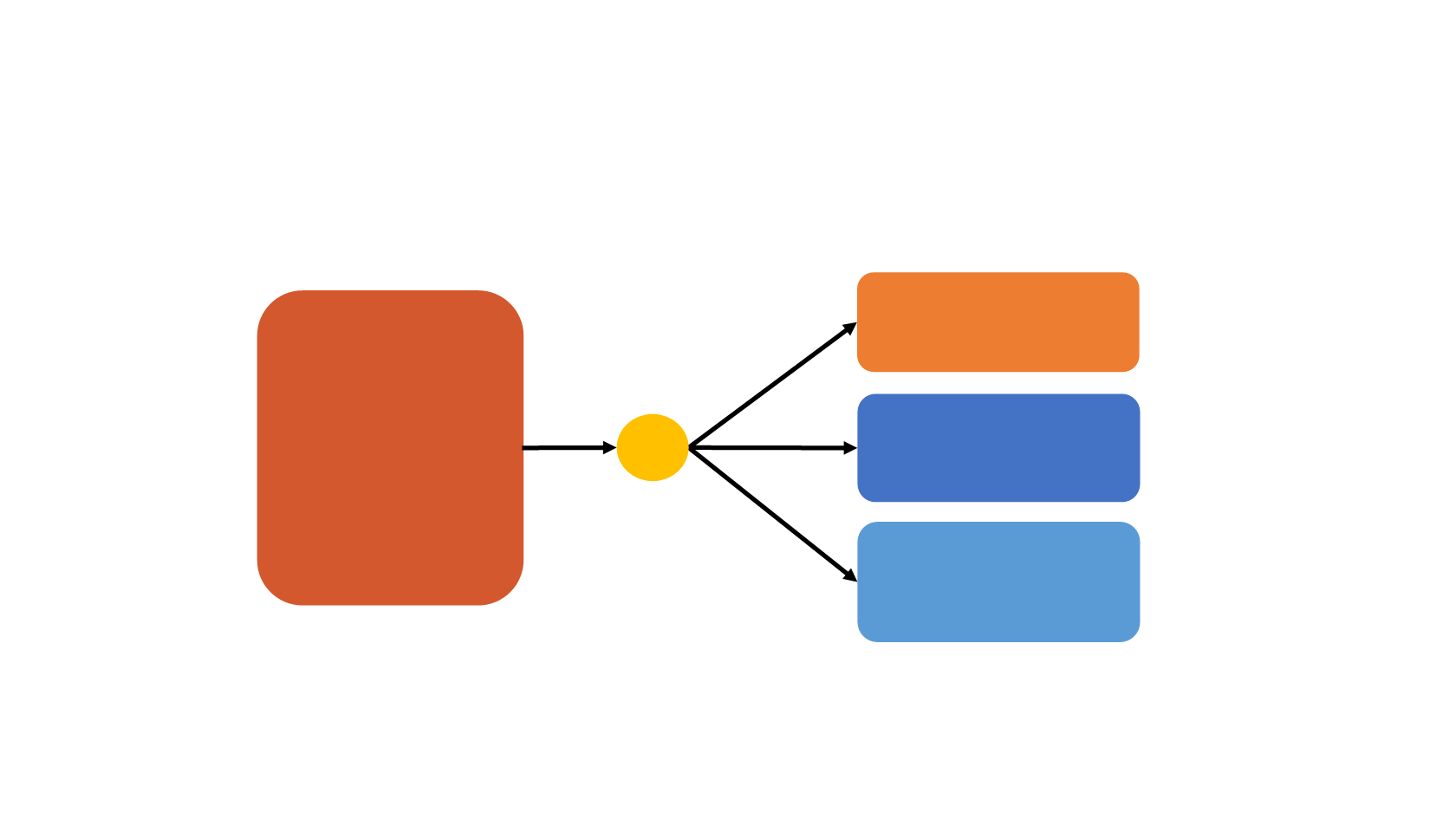
Randomized Phase 3 trial: MM-398 + 5-
FU/LV as 2
nd
-line therapy for MPC
(NAPOLI-1)
MM-398
(120 mg/m
2
Q3W)
n=151
MM-398 +
5-FU/LV
(80 mg/m
2
+ 2400 mg/m
2
over 46 h /
400 mg/m
2
Q2W)
n=117
1:1:1
5-FU/LV
(2000 mg/m
2
over 24 h / 200 mg/m
2
weekly Q6W)
n=149
•
MPC
•
Received prior
gemcitabine-based
therapy
•
N=417
R
Primary endpoint: OS
Secondary endpoints: PFS, ORR, CA19-9 response, safety
Stratification:
Albumin, KPS, ethnicity
Wang-Gillam et al. Lancet 2015. doi: 10.1016/S0140-6736(15)00986-1
Primary objective OS









