
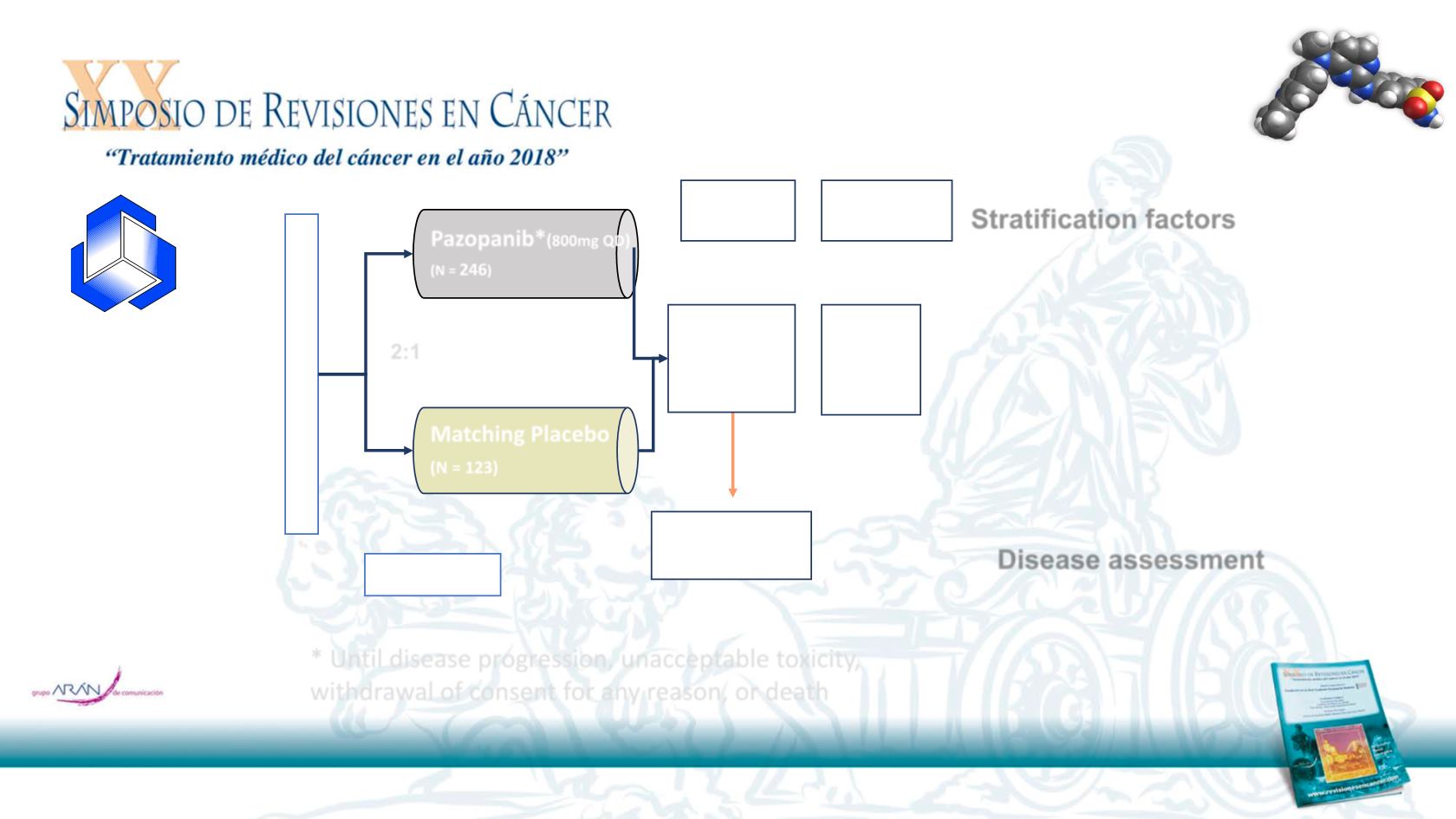
Phase III Study Design
* Until disease progression, unacceptable toxicity,
withdrawal of consent for any reason, or death
Matching Placebo
(N = 123)
R
A
N
D
O
M
I
s
E
2:1
Pazopanib*
(
800mg QD
)
(N =
246
)
N= 369
PFS
(RECIST v1.0)
OS
ORR
QoL
Safety
1
0
Endpoint
2
0
Endpoints
Followed for
survival
Stratification factors
Performanc
e status
(0 vs 1)
Number of prior lines of
systemic therapy for
advanced disease
(0/1 vs 2+)
Disease assessment
at week 4-8-12-20 and
at
8 week intervals
thereafter
Van Der Graaf W, et al. Lancet 2012;379:1879-1886









