
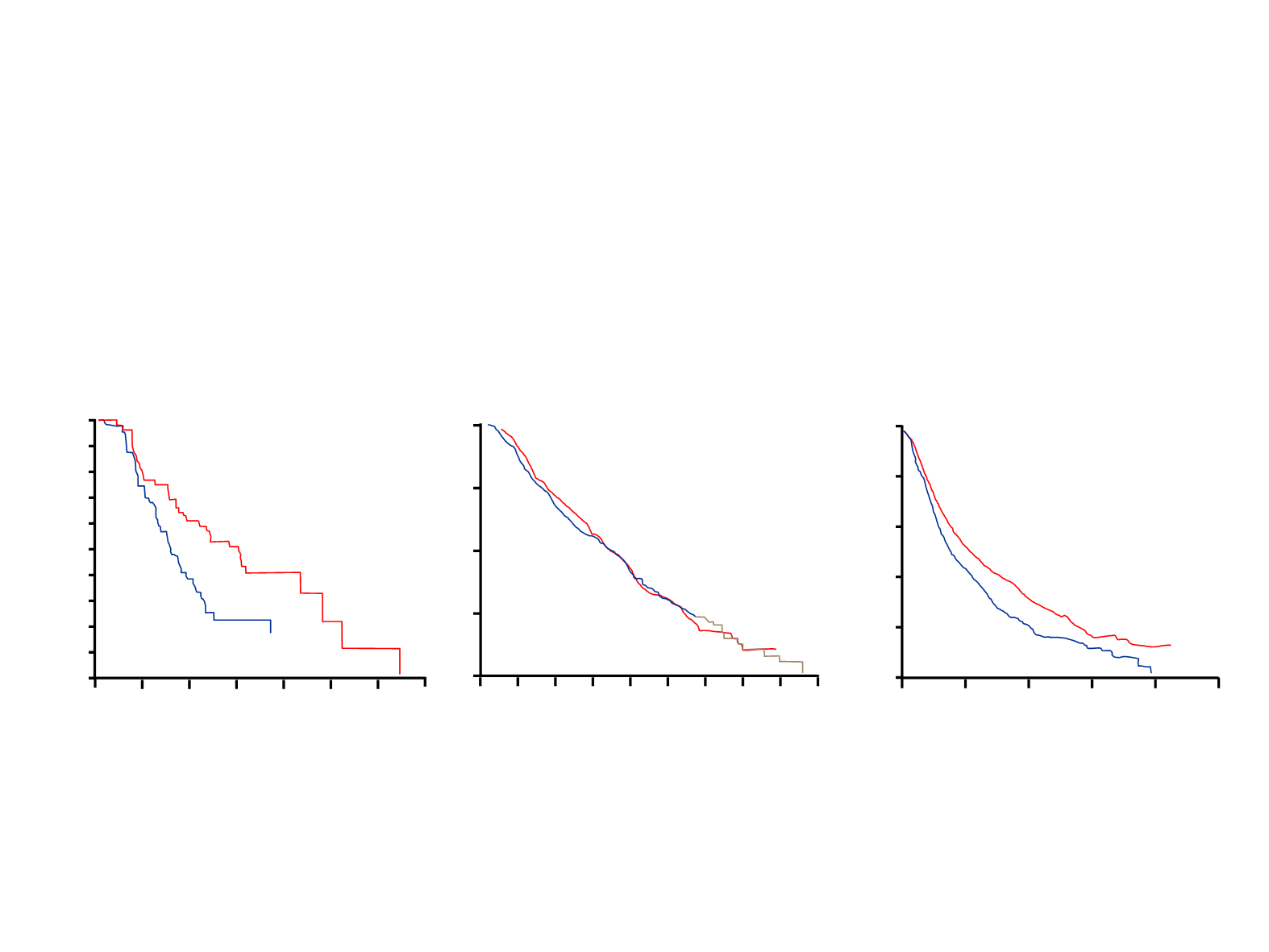
Note: Data are provided as a summary of different trials findings. Data pertaining to different trials cannot be compared due to inherent differences
in trial design and methodology.
1. Shepherd FA,
et al
.
J Clin Oncol
. 2000;18:2095-103;
2. Hanna N,
et a
l.
J Clin Oncol
. 2004;22:1589-97;
3. Shepherd FA,
et al
.
N Engl J Med
2005;353:123-32.
TAX 317B
1
JMEI
2
BR-21
3
Docetaxel
7.5 months
Pemetrexed 8.3 months
Erlotinib
6.7
BSC
4.6 months
Docetaxel
7.9 months
Placebo
4.7
P: 0.01
HR: 0.99 (0.82, 1.20)
HR: 0.70 (0.58, 0.85)
100
90
0
3
6
9
12
15
18
21
80
70
60
50
40
30
20
10
0
100
75
50
25
0
0.0
2.5
5.0 7.5 10.0 12.5 15.0 17.5 20.0 22.5
100
80
60
40
20
0
0
6
12
18
24
30
Survival probability
Survival (months)
Survival (months)
Survival (months)
Tratamiento estándar en 2L para
CPNM avanzado hasta 2014









