
90% power to detect a hazard ratio of 0.71 (improvement in median PFS from 10 months to
14.1 months)
Ramalingam ESMO 2017; Soria, NEJM 2017
Stratification
by
mutation
status
(Exon 19 deletion
/ L858R)
and
race
(Asian / non-
Asian)
Crossover was allowed for patients in
the
SoC
arm, who could receive open-
label osimertinib upon central
confirmation of progression and T790M
positivity
Patients with locally advanced or
metastatic NSCLC
Key inclusion criteria
•
≥18 years*
•
WHO performance status 0 / 1
•
Exon 19 deletion / L858R (enrolment by
local
#
or central
‡
EGFR testing)
•
No prior systemic anti-cancer /
EGFR-TKI therapy
•
Stable CNS metastases allowed
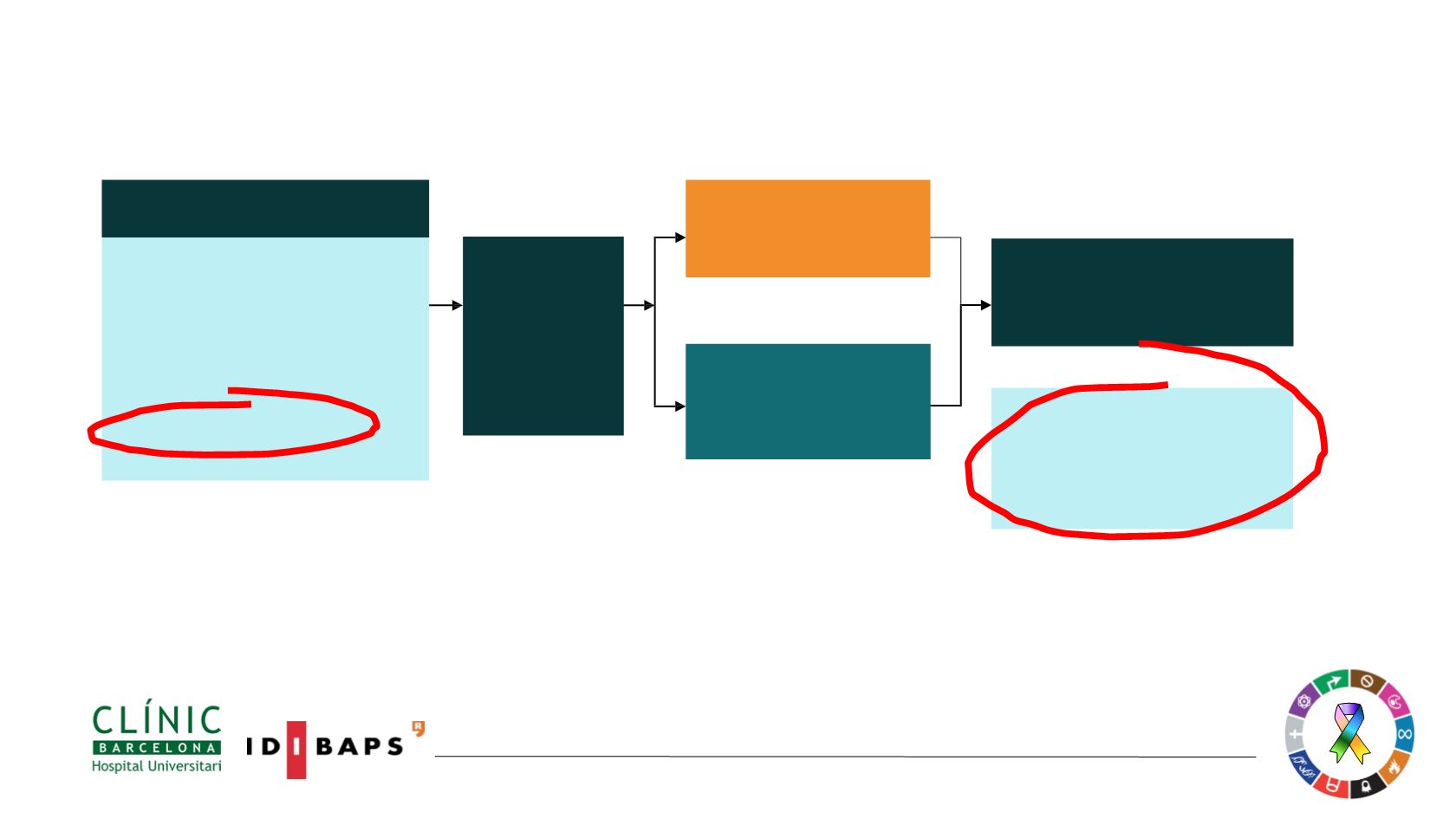
Randomised1:1
RECIST 1.1 assessment every 6
weeks
¶
until objective progressive
disease
EGFR-TKI SoC
§
;
Gefitinib
(250 mg p.o. qd) or
Erlotinib
(150 mg p.o. qd)
(n=277)
Osimertinib
(80
mg p.o. qd) (n=279)
OSIMERTINIB VS STANDARD-OF-CARE EGFR-TKI AS FIRST-LINE
TREATMENT IN PATIENTS WITH EGFRm ADVANCED NSCLC: FLAURA
Primary endpoint:
• Progression-free survival based on investigator assessment (RECIST 1.1)
Secondary endpoint
:
• ORR, OS, DCR, Duration of Response, PRO and safety









