
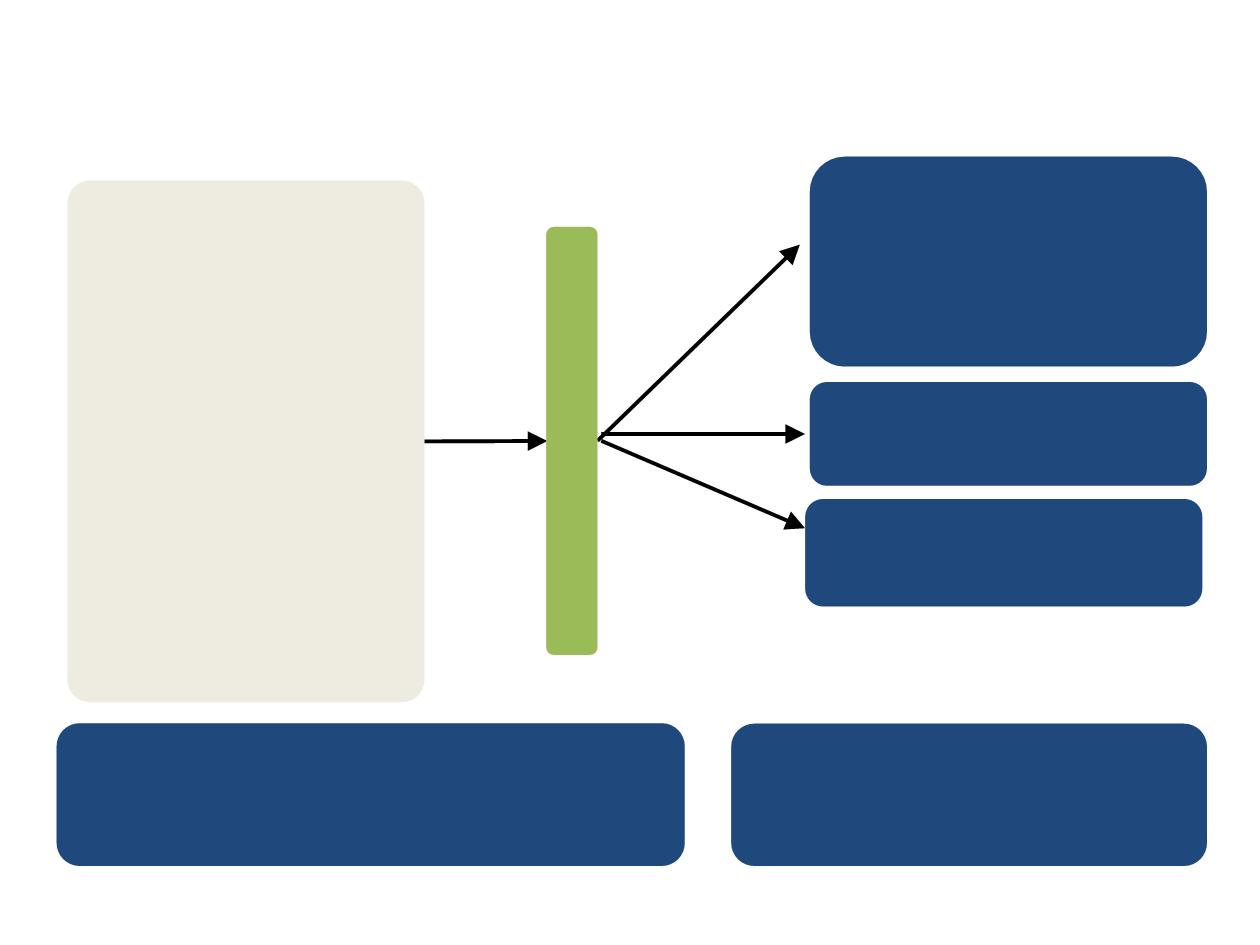
HOPE-205: Lenvatinib + Everolimus
Motzer RJ, et al.
Lancet Oncol.
2015;16:1473-82
(A) Lenvatinib
18 mg/day
+
Everolimus
5 mg/day
•
Histologically verified clear
cell RCC
•
Radiographic evidence of
progressive advanced or
mRCC within 9 months of
stopping previous treatment
•
One previous disease
progression with VEGF-
targeted treatment
•
ECOG PS 0−1
•
Adequately controlled blood
pressure
•
Adequate renal, bone
marrow, blood coagulation,
liver, and cardiac function
(B) Lenvatinib
24 mg/day
N=153
1:1:1
(C) Everolimus
10 mg/day
R
A
N
D
O
M
I
Z
A
T
I
O
N
Randomization stratified by: Hemoglobin (men, ≤130 vs.
>130 g/dL; women, ≤115 vs. >115 g/dL) and corrected
serum calcium (≥2.5 mmol/L vs.
<2.5 mmol/L)
Primary endpoint: PFS
Secondary endpoints: Safety/tolerability,
pharmacokinetics, OS, and ORR
Study Design









