
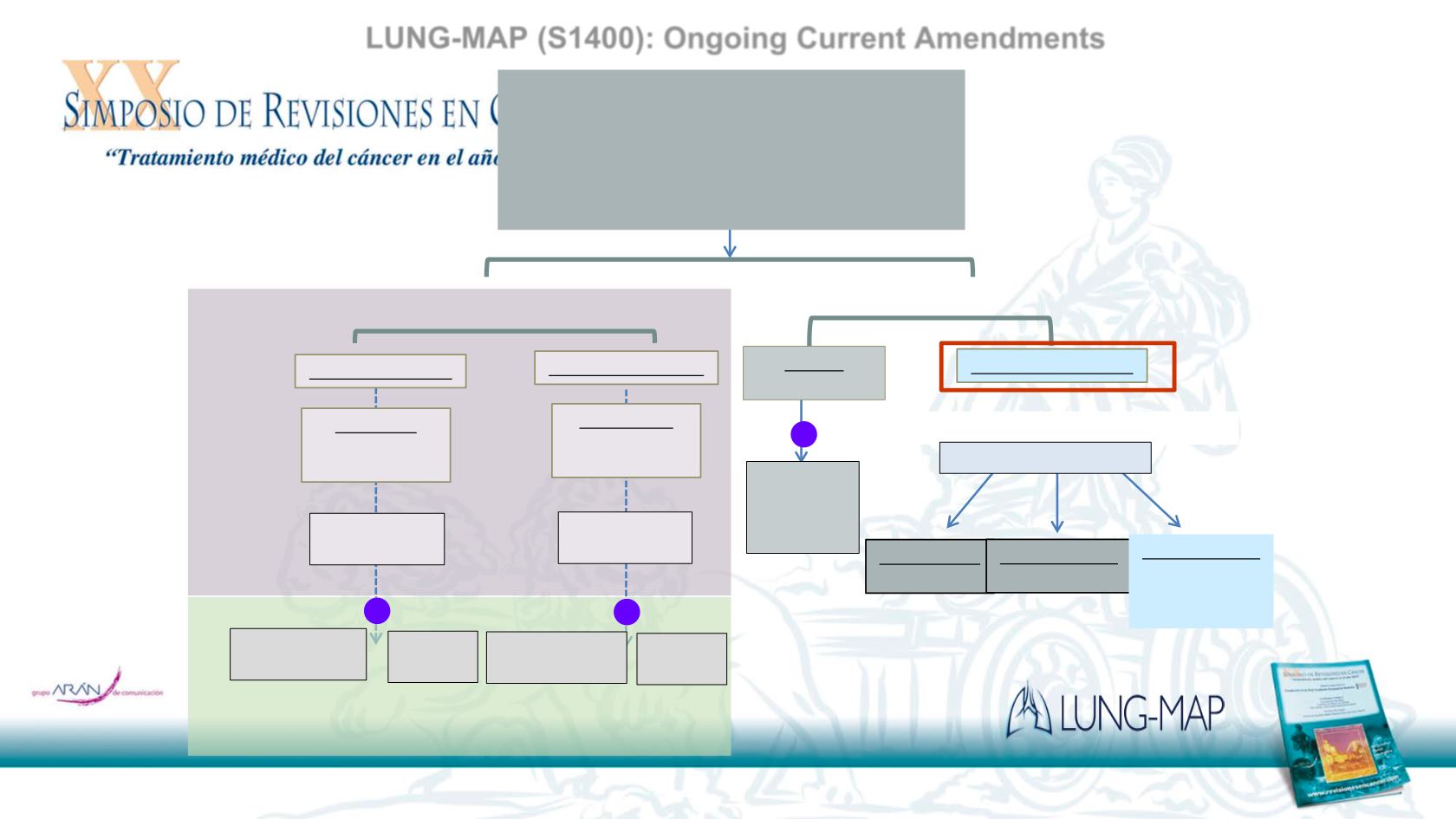
Biomarker 1 Positive
…Biomarker n Positive
…Sub-study n
Biomarker-driven
Therapy
Biomarker-matched
*
Sub-studies
Previously-treated Stage IV or Recurrent
Non-Small Cell Lung Cancer
(all histologies)
Immunotherapy or Chemotherapy
Relapsed/Refractory Patients
Sub-study 1
Biomarker-driven
Therapy
Stage 2:
Stage 1:
Investigational
therapy n
Standard
of Care
R
Investigational
therapy 1
Standard
of Care
R
Investigational
therapy 1
Investigational
therapy n
Collect tissue for Immuno-Biomarker Profiling
Non-Matched Sub-studies
IO Sub-study 1
IO combo 1
…IO Sub-study m
IO combo m
Common Control
Dealer’s choice
based on
histology
Randomization
IO Relapsed/Refractory
IO Naïve
(squamous only)
Nivolumab +
Ipilimumab
V.
Nivolumab
R
Slide # 45
LUNG-MAP (S1400): Ongoing Current Amendments









