
REPLAY TRIAL
SLCG
Multi-center exploratory phase II trial of Pembrolizumab (200 mg ) as second or
further line with NSCLC who have failed to a prior treatment with anti-PDL1 drug
Primary:
Overall Response
Rate (ORR) per
RECIST v1.1 and
irRC
Secondary:
•
Progression Free Survival (PFS)
per RECIST v1.1 and irRC
•
Overall Survival (OS) per RECIST
v1.1 and irRC
•
Safety
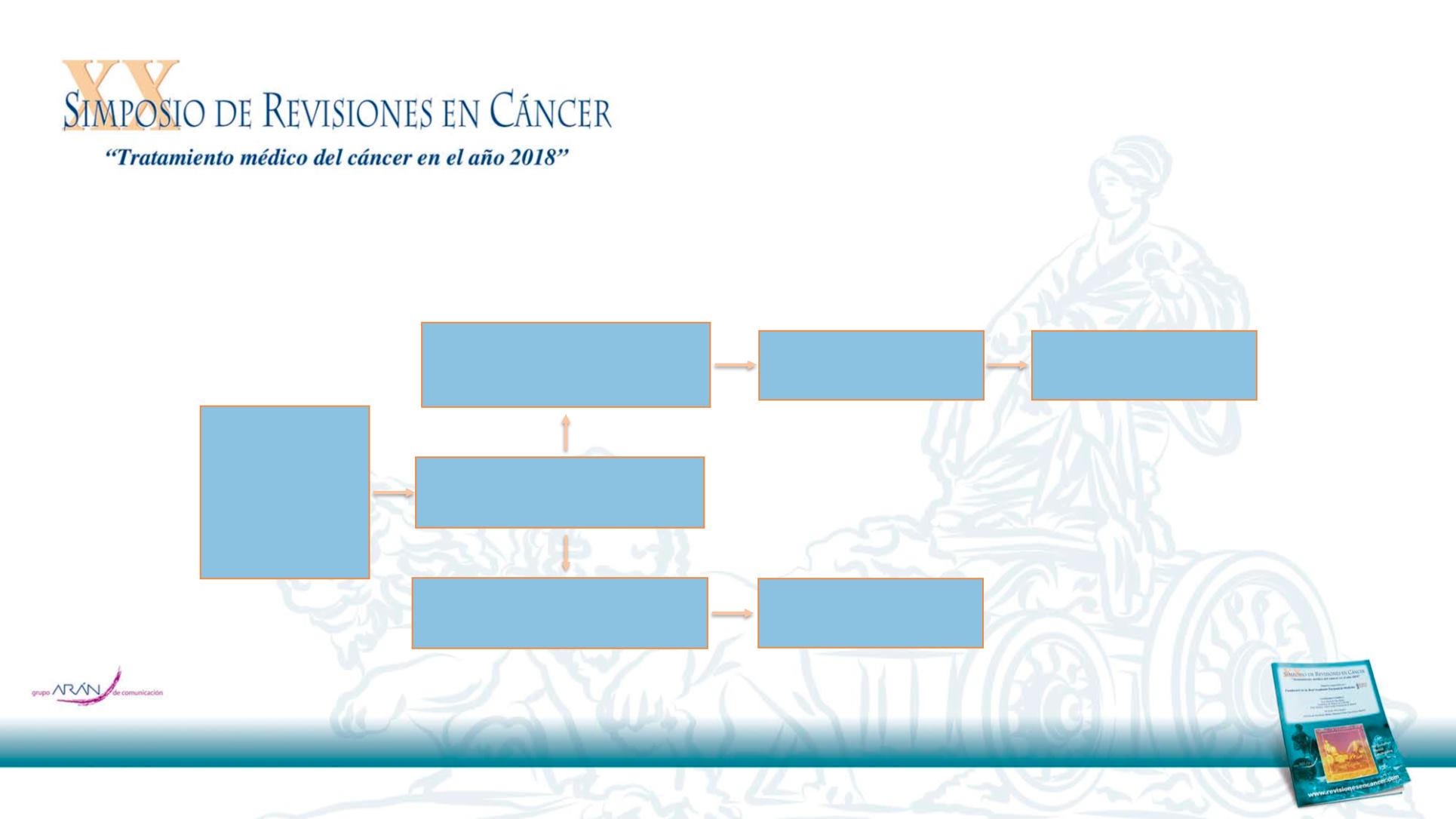
Advanced/metasta
tic NSCLC
≥2nd
line who have
failed prior
PD1/PDL1
checkpoint
inhibitor
Response or Stable Disease
for at least > 16 weeks
Experience PD while on treatment
OR PD < 12 weeks after stopping
treatment
Stop treatment and PD > 12 weeks
after stopping treatment
Re-Treatment with
Pembrolizumab
Chemotherapy ≥ 4 cycles
(free election for the PI)
Re-Treatment with
Pembrolizumab
PD
PD
PD
Cohort 2
Cohort 1
Bx
Bx
110 patients.
Up to 2 y.
Up to 2 y.
Courtesy Dr. S Ponce









