
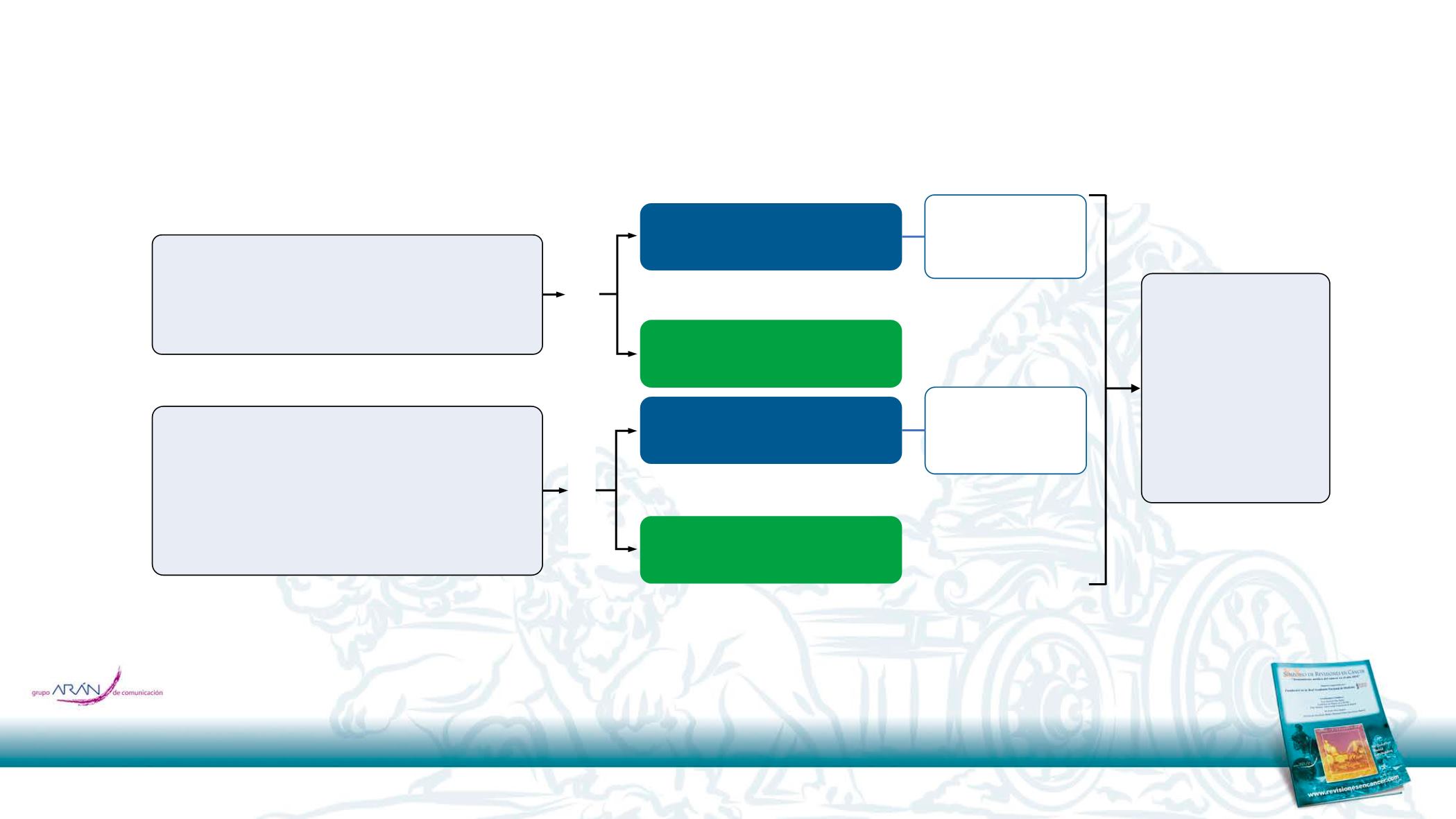
CheckMate 017 and 057 study designs
a
The protocols of both studies were amended in September 2016, when minimum follow-up was approximately 2.5 years, allowing patients to switch to nivolumab 480 mg Q4W starting 2 weeks after their last 3-
mg/kg Q2W dose;
b
After completion of the primary analyses,
3,4
patients in the docetaxel arms who ended treatment at any time during the studies were allowed to cross over to nivolumab
ALK = anaplastic lymphoma kinase; ECOG PS = Eastern Cooperative Oncology Group performance status; EGFR = epidermal growth factor receptor; IV = intravenous; LCSS = Lung Cancer Symptom Scale; ORR =
objective response rate; PFS = progression-free survival; Q3W = every 3 weeks; TKI = tyrosine kinase inhibitor
Nivolumab 3 mg/kg IV Q2W
until progressive disease or
unacceptable toxicity
(n = 292)
Docetaxel 75 mg/m
2
IV Q3W
until progressive disease or
unacceptable toxicity
b
(n = 290)
Nivolumab 3 mg/kg IV Q2W
until progressive disease or
unacceptable toxicity
(n = 135)
CheckMate 017
(NCT01642004; N = 272)
CheckMate 057
(NCT01673867; N = 582)
Key eligibility criteria
•
Stage IIIB/IV
SQ NSCLC
•
ECOG PS 0–1
•
1 prior platinum-based chemotherapy
•
Pretreatment (archival or fresh) tumor
samples required for PD-L1 analysis
Key eligibility criteria
•
Stage IIIB/IV
non-SQ NSCLC
•
ECOG PS 0–1
•
1 prior platinum-based chemotherapy
•
Pretreatment (archival or fresh) tumor
samples required for PD-L1 analysis
•
Prior maintenance therapy allowed
•
Prior TKI therapy allowed for known
ALK
translocation or
EGFR
mutation
Endpoints
•
Primary
‒ OS
•
Additional
‒ PFS
‒ ORR
‒ Efficacy by tumor PD-L1
expression
‒ Safety
‒ Quality of life (LCSS)
Optional switch to flat dose
nivolumab 480 mg Q4W
allowed after September 2016
a
Optional switch to flat dose
nivolumab 480 mg Q4W
allowed after September 2016
a
Docetaxel 75 mg/m
2
IV Q3W
until progressive disease or
unacceptable toxicity
b
(n = 137)
Randomized 1:1
Randomized 1:1
Felip E. et al. ESMO 2017 1301PD









