
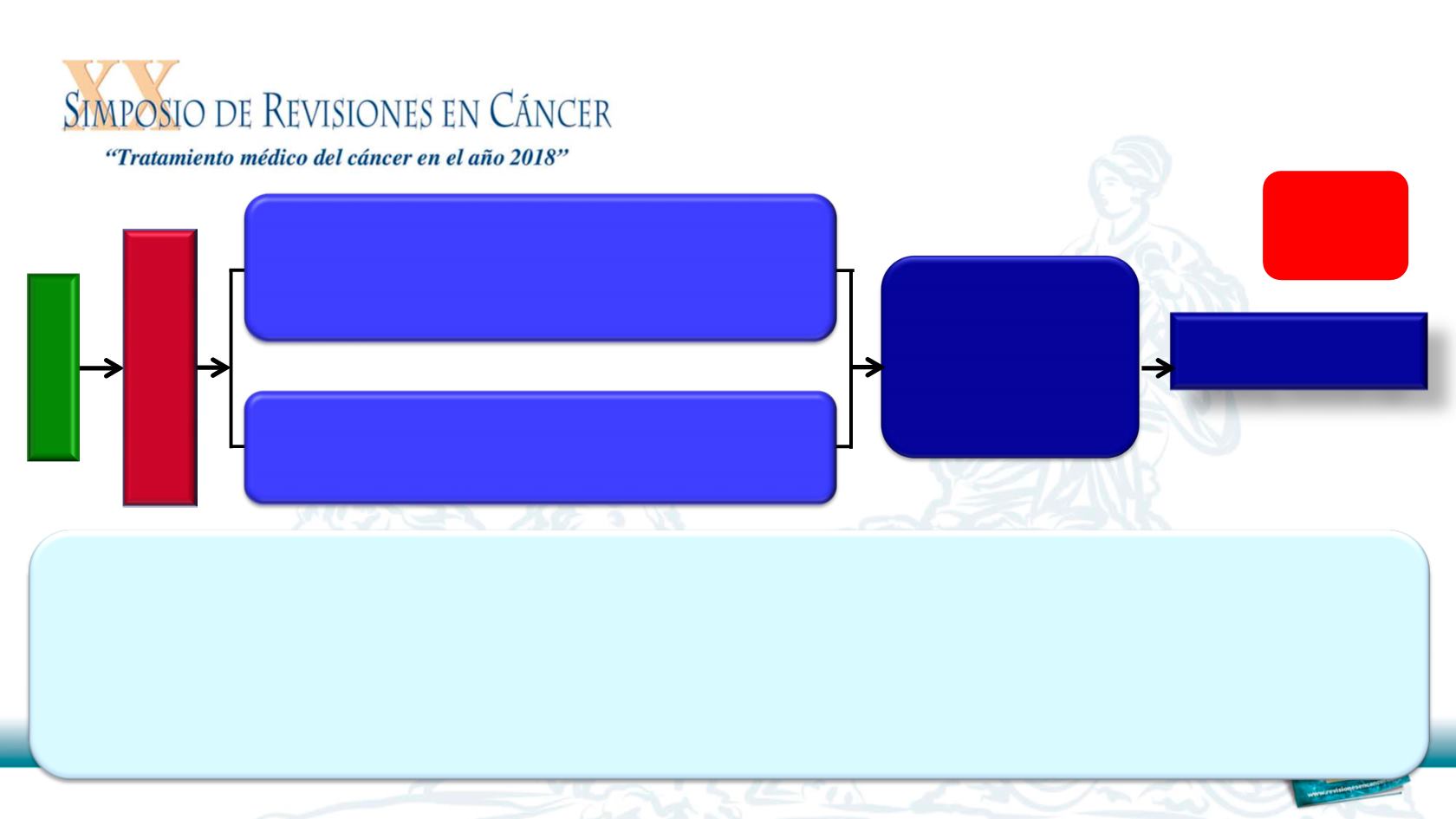
RAINBOW: Study Design
Treat until
disease
progression or
intolerable
toxicity
Ramucirumab 8 mg/kg day 1&15
+ Paclitaxel 80 mg/m
2
day 1,8 &15
of a 28-day cycle
N = 330
Placebo day 1&15
+ Paclitaxel 80 mg/m
2
day 1,8 &15
N = 335
R
A
N
D
O
M
I
Z
E
Survival and
safety follow-up
1:1
S
C
R
E
EN
•
Important inclusion criteria:
-
Metastatic or loc. adv. unresectable gastric or GEJ* adenocarcinoma
- Progression after 1
st
line platinum/fluoropyrimidine based chemotherapy
•
Stratification factors
:
-
Geographic region,
- Measurable vs non-measurable disease,
- Time to progression on 1
st
line therapy (< 6 mos vs. ≥ 6 mos)
OS









