
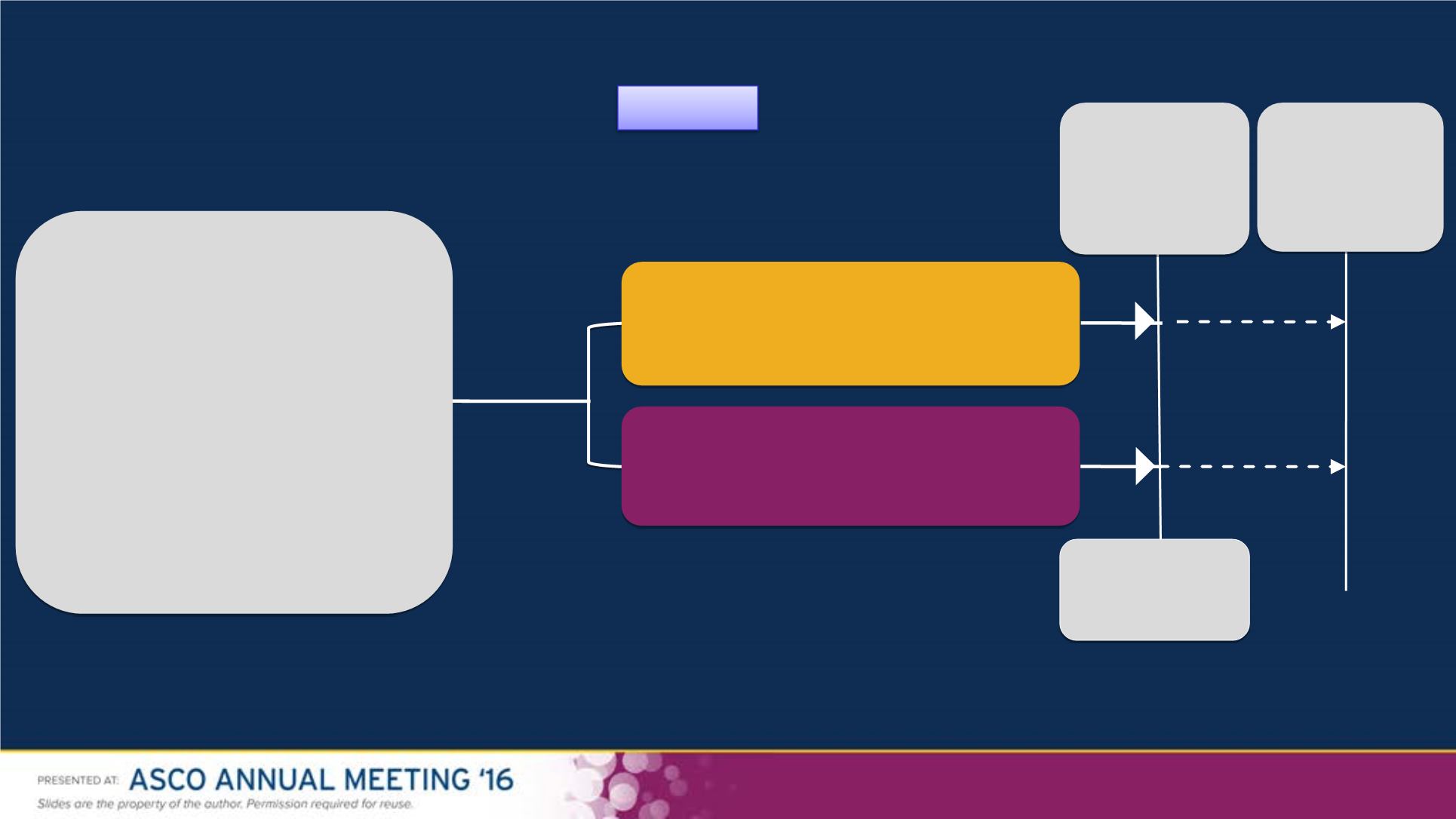
Study Design
Presented by: Keith T. Flaherty, MD
N = 947 screened
Primary Endpoint:
investigator-assessed PFS
Secondary Endpoints:
OS, overall response rate (ORR), duration of response, safety
N = 423
•
BRAF
V600E/K
• Unresectable stage IIIC/IV
• Treatment naive
• ECOG PS 0/1
• No brain metastases, unless:
Treated
Stable ≥ 12 weeks
Stratification
•
BRAF
-mutant (V600E vs K)
• LDH (> ULN vs
≤
ULN)
Dabrafenib + trametinib
150 mg BID + 2 mg QD
n = 211
Dabrafenib + placebo
150 mg BID + placebo QD
n = 212
Pre-planned
interim OS
[95 events]
Primary Analysis
(PFS)
[213 events]
Aug 2013
Final Analysis
(OS)
[222 deaths]
Jan 2015
BID, twice daily; ECOG PS, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase; QD, once daily; ULN, upper limit of normal.
NP4: 1606042435
COMBI-d
COMBI-d









