
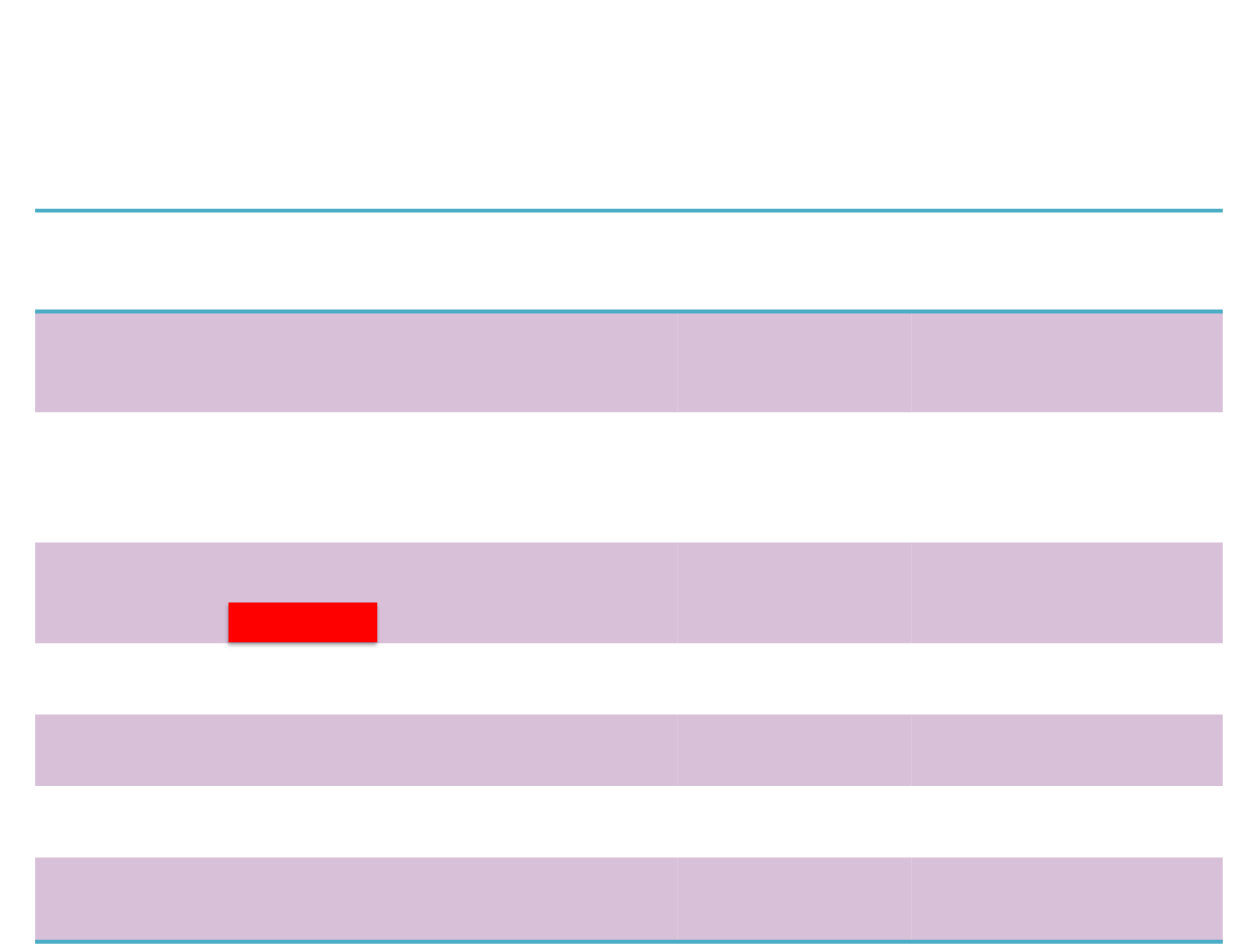
Ongoing Studies
in SCLC
Title
ClinicalTrials.gov
Identifier (Last
Updated)
Recruitment
Phase
Rovalpituzumab Tesirine as Maintenance Therapy Following
1st-Line Platinum-Based Chemo in Participants With Extensive
Stage SCLC (
MERU
)
NCT03033511
(July 31, 2017)
Recruiting
3
Rovalpituzumab Tesirine vs Topotecan in Pts With
Advanced/Metastatic SCLC With High Levels of DLL3 and Who
Have 1st Disease Progression During/Following Front-Line
Platinum-Based Chemotherapy (
TAHOE
)
NCT03061812
(July 31, 2017)
Recruiting
3
Rovalpituzumab Tesirine (SC16LD6.5) for 3rd-Line and Later
Treatment in Pts With Relapsed/Refractory DLL3-Expressing
SCLC (
TRINITY
)
NCT02674568
(June 15, 2017)
Active: not
recruiting
2
Safety and Tolerability of Rovalpituzumab Tesirine in Japanese
Pts With Advanced, Recurrent SCLC
NCT03086239
(July 31, 2017)
Recruiting
1
Rovalpituzumab Tesirine to Study Cardiac Ventricular
Repolarization in Subjects With SCLC
NCT02874664
(July 7, 2017)
Recruiting
1
Rovalpituzumab Tesirine (SC16LD6.5
)
in the Frontline
Treatment of Pts With DLL3-Expressing Extensive Stage SCLC
NCT02819999
(July 27, 2017)
Recruiting
1
Rovalpituzumab Tesirine in Combination With
Nivolumab ±
Ipilimumab
for pretreated Extensive-Stage SCLC p. (
M16-300)
NCT03026166
(July 31, 2017)
Recruiting
1
ASCO 2018









