
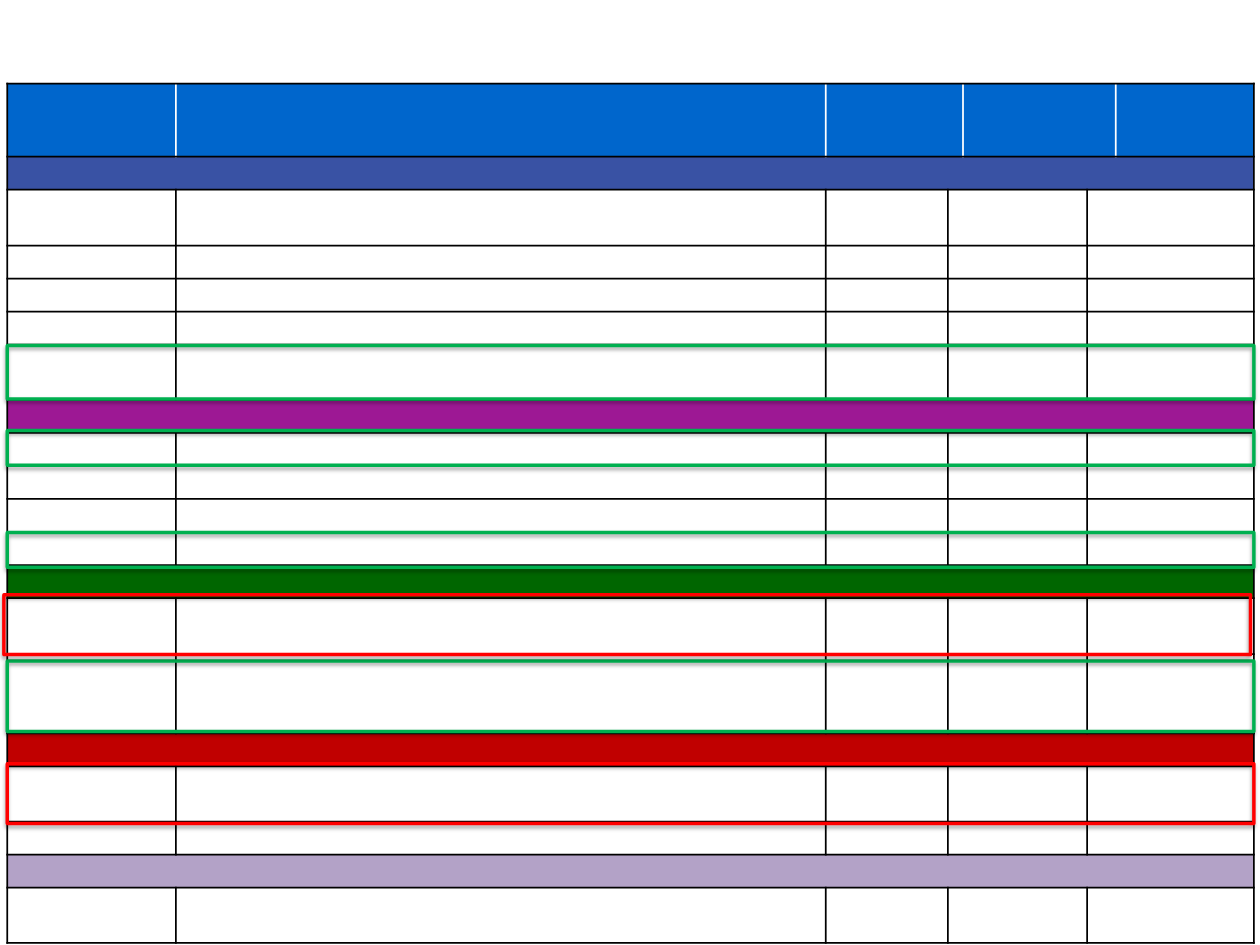
There are several ongoing phase III studies of anti-PDL1/PD1 therapy in first-line NSCLC
Study name
Study description
Monotherapy
Chemotherapy
combination
Anti-CTLA4
combination
Atezolizumab
IMpower110
Atezolizumab monotherapy (squamous and non-squamous)
TC1/2/3 or IC1/2/3 (TC or IC ≥1%)
IMpower130
Atezolizumab + platinum doublet chemotherapy (non-squamous)
IMpower131
Atezolizumab + platinum doublet chemotherapy (squamous)
IMpower132
Atezolizumab + platinum doublet chemotherapy (non-squamous)
IMpower150
Atezolizumab + platinum doublet chemotherapy ± bevacizumab (non-squamous)
Pembrolizumab
KEYNOTE-024
Pembrolizumab monotherapy (squamous and non-squamous)
TPS ≥50%
KEYNOTE-042
Pembrolizumab monotherapy (squamous and non-squamous)
TPS ≥1%
KEYNOTE-407
Pembrolizumab + platinum doublet chemotherapy (squamous)
KEYNOTE-189
Pembrolizumab + platinum doublet chemotherapy (non-squamous)
Nivolumab
CheckMate 026
Nivolumab monotherapy (squamous and non-squamous)
TC ≥1% (TC ≥5% as co-primary endpoint)
CheckMate 227
Nivolumab monotherapy or + ipilimumab or + platinum doublet chemotherapy
(squamous and non-squamous)
PD-L1+ only for monotherapy arm
Durvalumab
MYSTIC
Durvalumab monotherapy or + tremelimumab (squamous and non-squamous)
NEPTUNE
Durvalumab + tremelimumab (squamous and non-squamous)
Avelumab
JAVELIN Lung 100
Avelumab monotherapy (squamous and non-squamous)









