
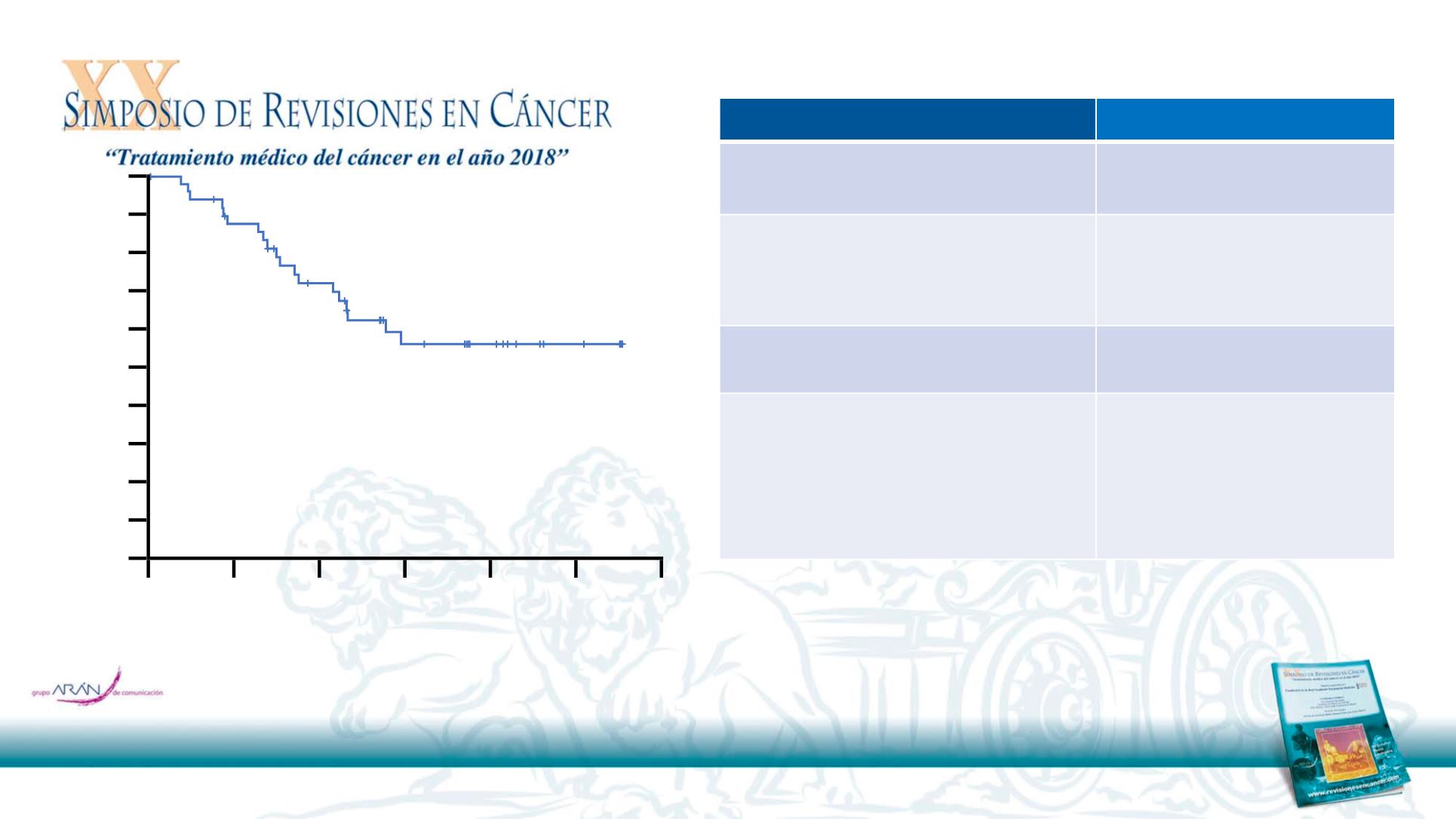
Clinically meaningful efficacy in the CNS
CNS PFS by BICR
Total (n=50)
Median follow-up for
CNS PFS*
11.2 months
CNS progression or death
#
Maturity
19 / 50
38%
Median CNS PFS
#
, months
NC (95% CI 7, NC)
Progression-free
at 6 months
§
at 12 months
§
72% (95% CI 57, 83)
56% (95% CI 40, 70)
CNS progression or death events that do not occur at the time of analysis are censored
Time from first dose (months)
Probability of
progression-free survival
No. at risk
100
90
80
70
60
50
40
30
20
10
0
0
3
6
9
12
15
18
50
41
31
18
13
4
Progression-free survival (n=50)
•
At 9 months, 75% (95% CI 53, 88) of patients were
estimated to remain in CNS response without
progression or death
Goss Ann Oncol 17









